- A state variable is an observable property of a system, such as temperature, volume, and pressure.
- The set of state variables that describes a system in its current condition defines the system's thermodynamic state.
- Extensive state variables scale with the size of the system, while intensive state variables do not.
- The mathematical relationships between state variables are described using an equation of state.
- Equations of state only hold at equilibrium.
- Equilibrium describes a reversible process in which the rate of the reactions in the forward and reverse directions are identical, so that the system gives the appearance of having a static composition (adapted from the IUPAC Gold Book).
- An ideal gas is a collection of gas-phase (low density) point-particles (with mass but no volume) that are non-interacting (no forces between particles except during collisions) and are in constant, random motion. When ideal gas particles collide, they undergo perfectly elastic collisions (i.e. momentum is conserved and no energy is lost to deformations or friction).
- The ideal gas law,
, is an equation of state that shows how the pressure (P), volume (V), number of moles (n), and the temperature (T) of an ideal gas system relate to each other at equilibrium. R is the universal gas constant.
The macroscopic properties of a thermodynamic system are described using state variables (also called state functions). These are observable quantities such as temperature, volume, and pressure that can be measured without changing the state of the system. The thermodynamic state is a quantitative description of the system using a set of state variables.
For example, we can measure the temperature, volume, mass, and pressure of a sample of water (the system) inside a glass. We can then describe the state of the water. If we alter the water, for instance by increasing the temperature by 10 degrees, then our system would be in a different thermodynamic state. A system can only be in one state at a time, but it can go through many different states over time.
A feature of thermodynamics is that the properties of a system in one particular state will be the same regardless of how, when, or where the state was achieved. For example, any 200 g sample of pure water at T = 25 °C and P = 1.0 bar at equilibrium will occupy the same volume, have the same density, and will contain the same amount of energy as the water above in state 1.
Suppose that we had a bucket of water at T = 298 K and P = 1.0 bar. If we remove 10.0 g of water from the bucket as sample 1 and 20.0 g of water as sample 2, we would find that some of the measured state variables would be different between the two samples while others would be the same. For example, the temperatures and pressures are the same, but the masses are different. The volumes would be different, but the densities would be the same.
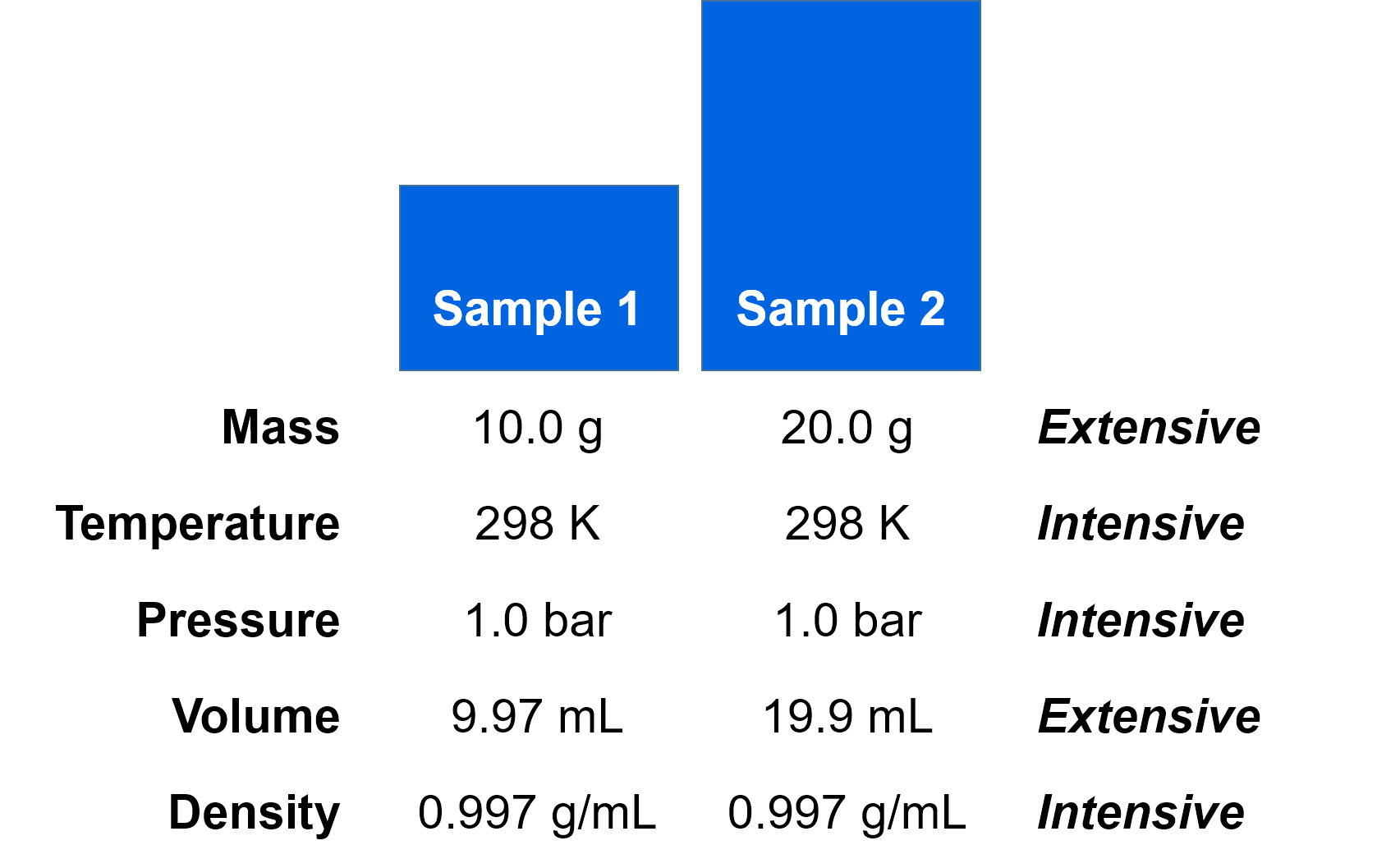
Based on these observations, we can classify state variables into two different categories. State variables that depend on the size of the system are called extensive state variables. For example, the two different sizes of water samples above (10.0 g vs 20.0 g) had different masses and volumes, and thus mass and volume are extensive properties. Extensive properties are proportional to the amount of material, so scale with the size of the system.
The second category of state variables, which do not depend on system size, are called intensive state variables. Intensive state variables depend on the nature of the material, but not the amount of material present. For example, the temperatures, pressures, and densities were the same for the two samples above despite the fact that the samples were different sizes. Thus, temperature, pressure, and density are classified as intensive properties. Two different samples from the same system (e.g. our 10.0 g and 20.0 g water samples from the same bucket) will always have the same set of intensive state variables, even if the samples don't have the same size.
Avoid this common error when categorizing state variables
Doubling the system size does not mean that we keep the same mass in twice the volume. Doubling the volume only (without also doubling the mass) will put the system into a different state (the pressure would be lower). We want to see how changing the size of the system will affect the state variables, but we must keep the system in the same state.
The system size dependence can be eliminated by dividing one extensive variable by another. This is one way to “cancel” the size-dependence. For example, density is defined as mass divided by volume. Both mass and volume are extensive state variables, but density is an intensive state variable. While the masses of the two water samples in the example above are obviously different, the densities of the two samples will be identical.
Avoid this common error when dividing intensive state variables
While we can eliminate the system size-dependence by dividing one extensive variable by another, we cannot introduce system size-dependence by dividing one intensive variable by another. If we took the ratio of the temperature over the pressure, T/P, (for whatever reason), it would not change with the system size, because neither T nor P would change. The ratio of two intensive properties is still an intensive property.
For state variables to accurately describe a thermodynamic system, the system must be in equilibrium. At equilibrium, the properties of the system (state variables such as T, P, V) do not vary in time. The equilibrium values of the state variables such as T, P, and V are related by precise mathematical equations called equations of state. Equations of state can be straightforward (common for simple systems like an ideal gas, which we will see shortly), complex, or unknown. Equilibrium is a very important concept in chemistry and biology.
Equations of state are only valid for a system at equilibrium. If a system is not in equilibrium, then the mathematical expression will not describe the physical system. This is okay! There is a large field of research that investigates non-equilibrium processes and phenomena. In this course, we are going to restrict our analysis to processes that start and end in equilibrium states.
The Ideal Gas Model
The ideal gas model is a very convenient first thermodynamic system to study. The gas phase is the simplest phase to study, and the thermodynamic results obtained from considering an ideal gas can be (carefully) generalized to more complicated systems.
The ideal gas model assumes that a collection of gas-phase (low density) point-particles have mass, but no volume. The particles are non-interacting (i.e. there are no forces among particles except during collisions) and in ceaseless, random motion. When ideal gas particles collide, they undergo perfectly elastic collisions (momentum is conserved; there is no energy loss to deformations or friction).
When a sample of ideal gas is in equilibrium, the values of the state variables are governed by an equation of state called the ideal gas law.
The ideal gas law shows how pressure, volume, the number of moles, and the temperature of the system are related at equilibrium:
where P is the pressure, V is the volume, n is the number of moles (mol) of gas, R is the Universal Gas constant, and T is the temperature (always in Kelvin, K). If the pressure is measured in atmospheres (atm) and the volume is measured in litres (L), then the most useful form of the Universal Gas constant is so that all of the units cancel appropriately.
The ideal gas law works very well for gases at high temperatures and low densities (i.e. low pressures). It does not work (at all) for substances that are not in the gas phase (e.g. solids or liquids), and it is not accurate when the interactions (attractions or repulsions) among the gas particles are too strong to be ignored.
Interactive: