You have previously learned that molecules are made up of atoms bonded together. But what does a molecule actually look like? Using the most powerful atomic microscopy techniques (see below), you can’t see much of the molecule – just a cloud of electrons surrounding the nuclei. For example, methane might look something like the model below.

You wouldn’t be able to see, for example, how the individual atoms interact nor their individual nuclei. But it is these interactions, and how they change, that we are interested in when we study chemical reactions. Thus, we need to be able to represent molecules in ways that can show us the individual atoms and their interactions. In this section we will study a number of different representations used to visualize molecules: Lewis dot structures, Kekule structures, line-bond structures, and wedge-dash structures. Chemists tend to use whichever representation is quickest/simplest, but still provides sufficient information for whatever they are trying to demonstrate.
Even with an incredibly powerful light microscope, you wouldn't be able to see a molecule because the wavelength of visible light is larger than the components that make up molecules (electrons, protons and neutrons). In recent years we have been able to actually visualize molecules at the atomic level. To image these molecules, one technique scientists use is called Atomic Force Microscopy (AFM). AFM works similarly to an old fashioned record player. In AFM, the point of the 'needle' (probe) is so fine that it’s made up of a single molecule of carbon monoxide (CO). When this CO probe is dragged across the surface of a molecule, the 'needle' is forced up whenever the electrons from the CO probe repel electrons in the molecule. The result is a picture of where the electrons are concentrated in a molecule. The fascinating picture below shows how similarly molecules look to the molecular models you see in this and other textbooks.
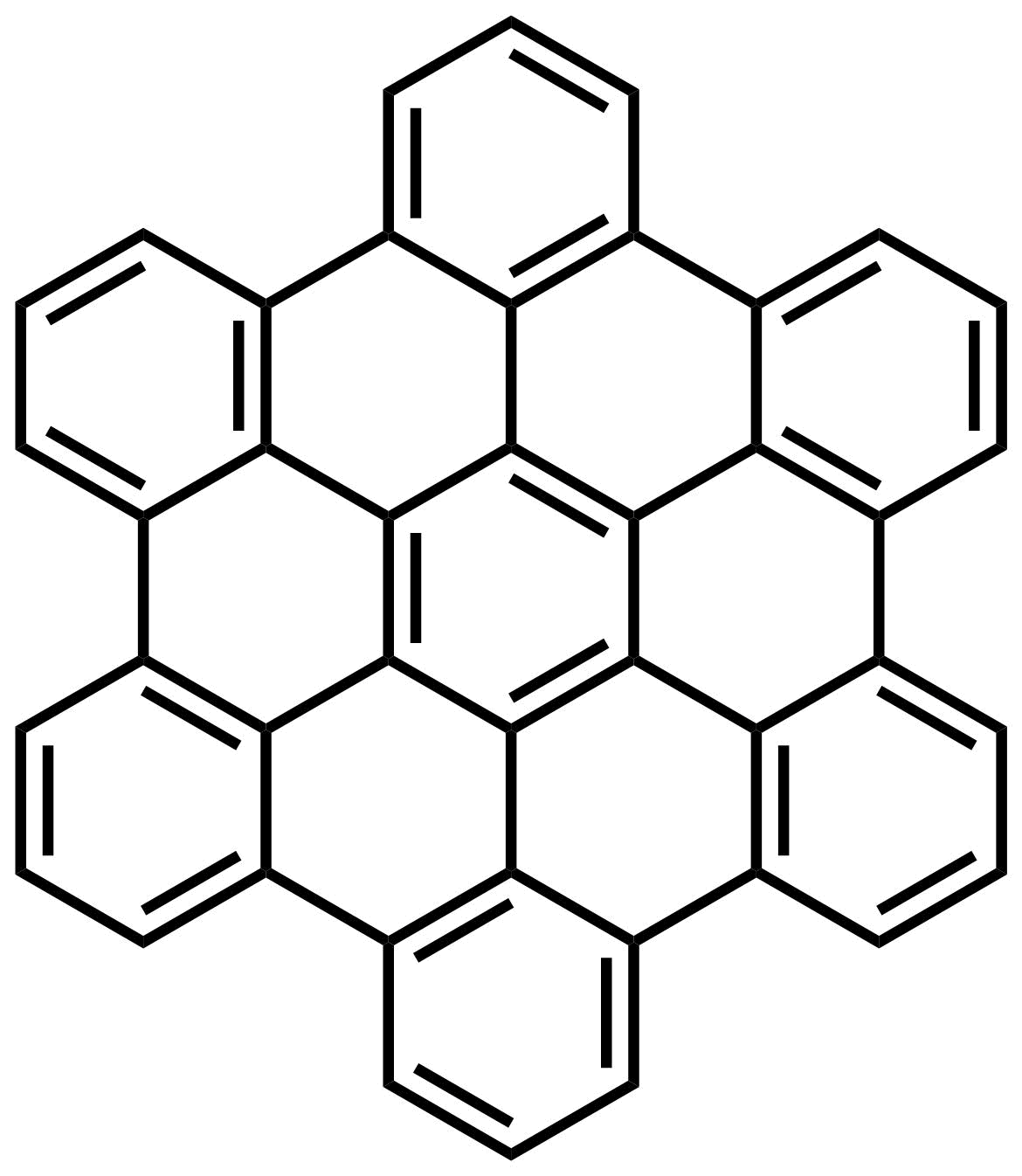 |
 |
| Structure of hexabenzocoronene | AFM image |