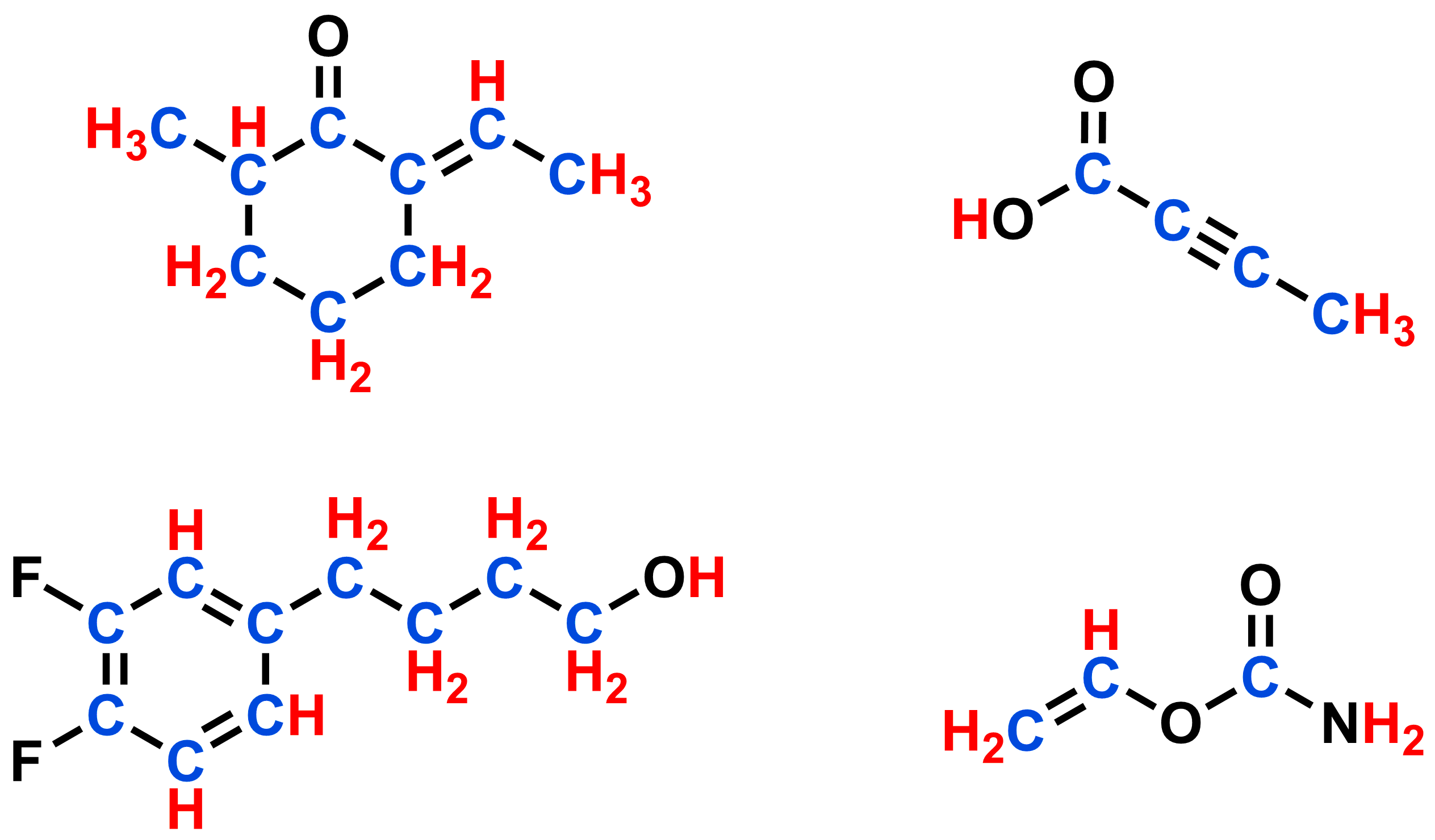
- Line bond structures can show large organic molecules efficiently
- The ends and bends of line bond structures are understood to be C atoms bonded to enough H atoms to complete the octet
- Elements other than C and H are always labelled explicitly
- H atoms bonded to atoms other than C are shown explicitly
- Formal charges are always included
- Lone pair electrons are often included, but are optional
Organic molecules can include many carbon and hydrogen atoms. Drawing out the atomic symbols, electrons, and bonds for each of these atoms every time we draw a molecule is not only time consuming, but can also make for a crowded and confusing diagram. We can use a short-hand, called a line-bond structure, to represent a molecule without having to draw each component explicitly. To convert the Kekule structure below to a line-bond structure, we make a number of simplifications.
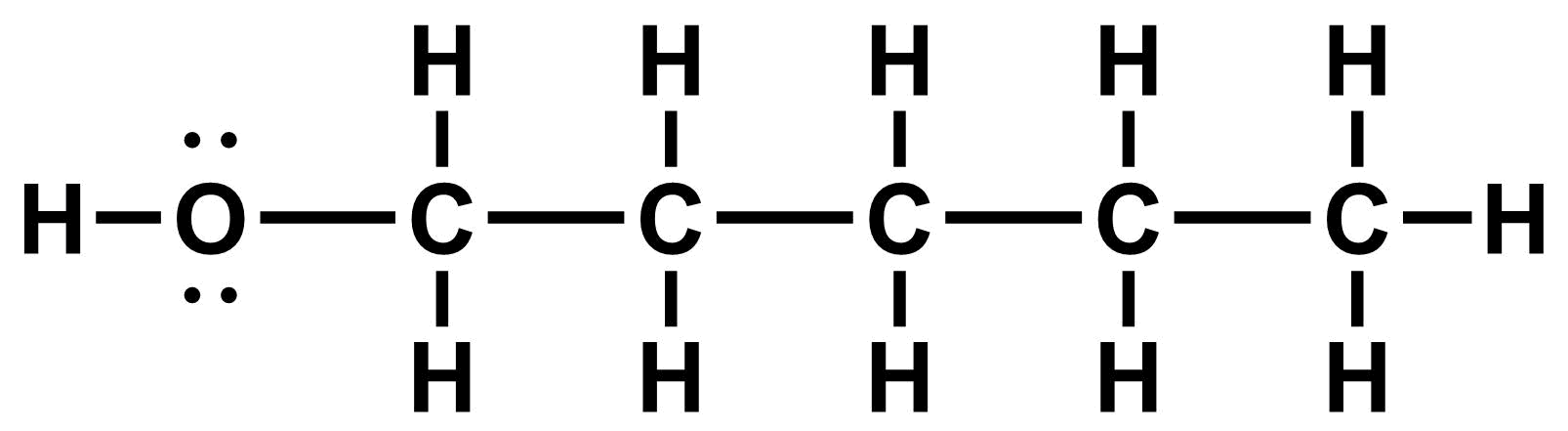
- Line-bond structures more accurately reflect the geometry of a molecule (see model below).

Our first step is to redraw the Kekule structure to better reflect this geometry: single and double bonds in the main chain are drawn as a zig-zag (109°-120° angles) and triple bonds are drawn linearly (180° angles).

- Next, remove the carbon atom labels. Each vertex (i.e. bend of a line) is understood to be a carbon atom.

- Finally, we don't draw in hydrogen atoms that are bonded to carbon. Instead, we understand that each carbon atom is bonded to as many hydrogen atoms as is needed to give the (neutral) carbon atom four bonds (i.e. a full octet).

Note that only hydrogen atoms bonded to carbon are implied. We must still explicitly draw in hydrogen atoms bonded to other atoms, such as in the HO group on the structure above.
Please note that lone pair electrons are sometimes left out of line-bond structures. Formal charges, however, are always included, so you can determine how many lone pair electrons are present even when they're not drawn in explicitly.
Click here to review formal charges
Rearrange the formula that we used to calculate formal charge. We know that:
and that
Thus, the number of lone pair electrons is given by:
Interactive:
Expand this section for detailed solutions to all 4 problems (try all 4 first!)