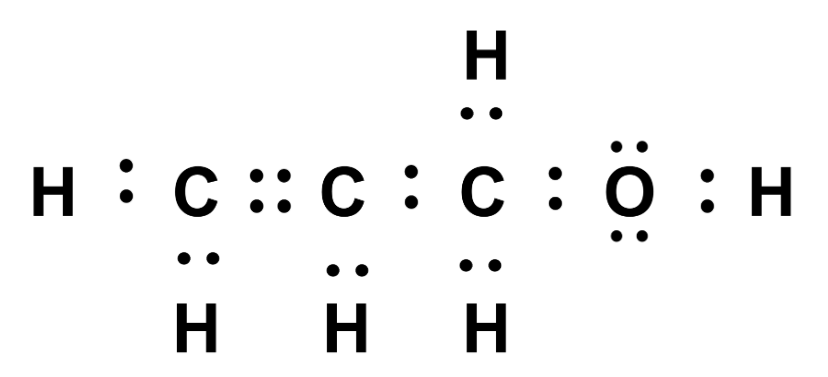
- Lewis dot structures use dots to represent electrons that are found in bonds and lone pair electrons
Let’s start with one of the simplest representations: Lewis dot structures. A Lewis dot structure is a diagram of a molecule that shows all bonding and lone pair electrons as dots. Lewis dot structures are useful when we’re focused on how the electrons in a molecule are arranged and which atoms are connected, but when we aren’t concerned about the shape of the molecule. Chemical reactions involve the outermost (valence) electrons, so we only consider these electrons when constructing Lewis dot structures.
Below are several examples of Lewis dot structures. Each element is represented by its atomic symbol, and each valence electron is represented by a single dot. Dots placed between two atoms represent bonding electrons, while dots isolated on a single atom represent lone pairs of electrons.
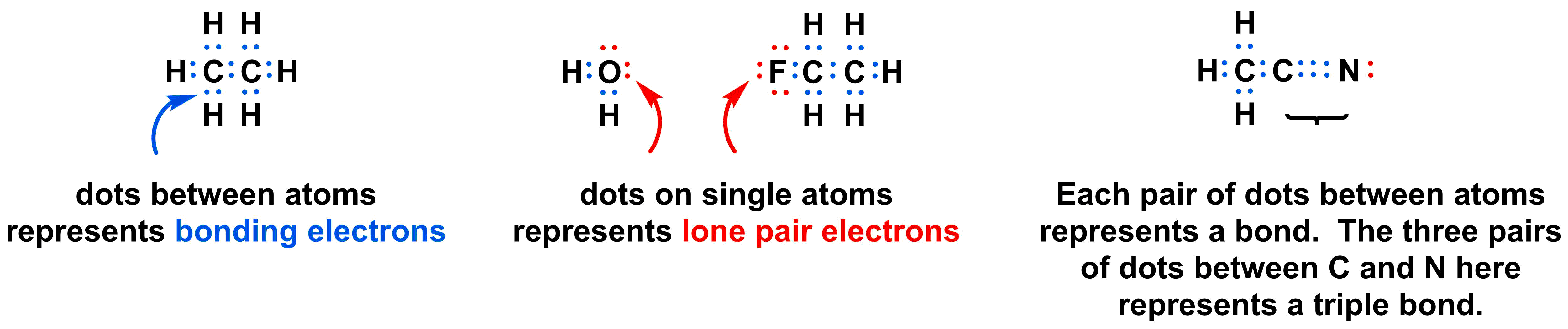
Constructing Lewis dot structures
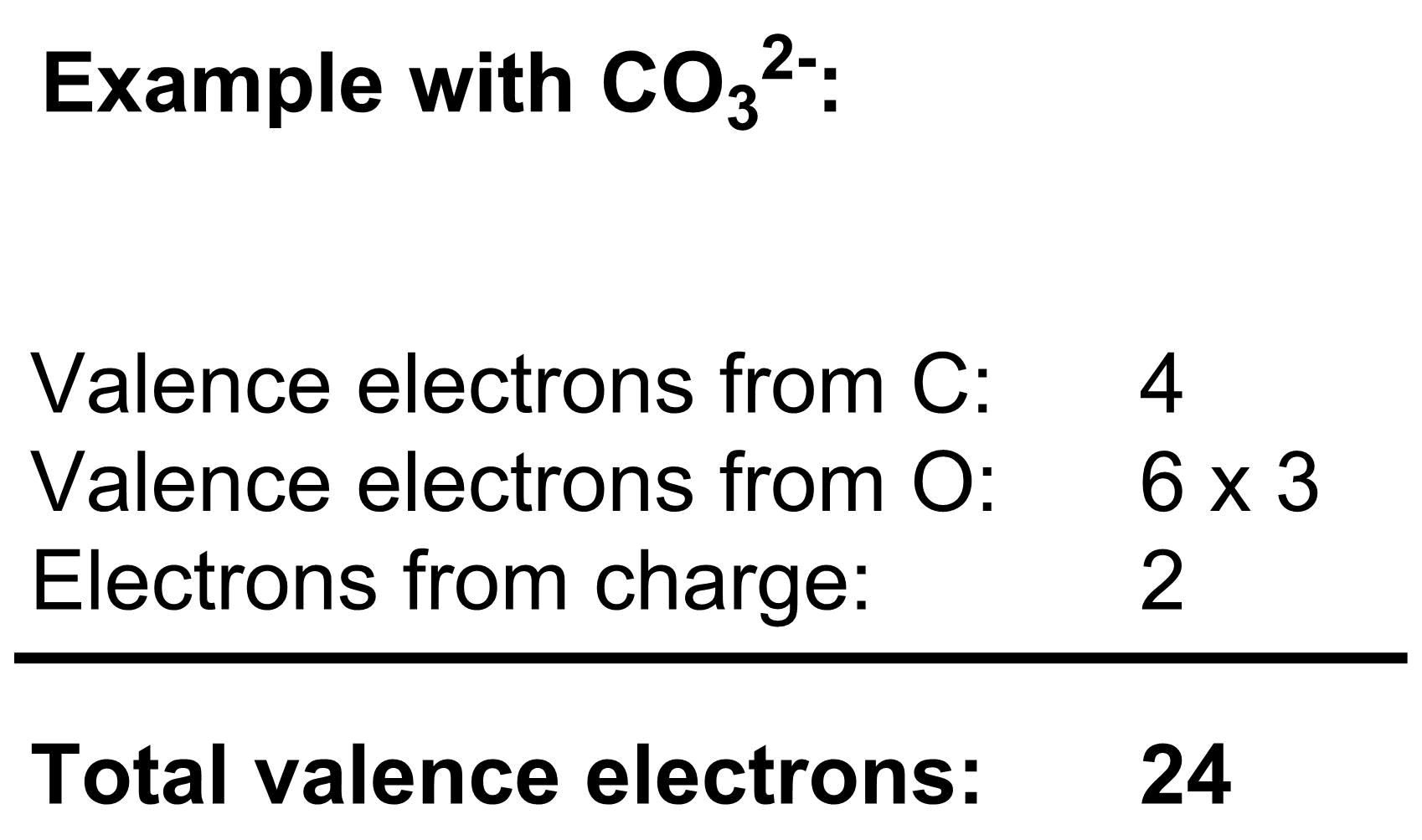
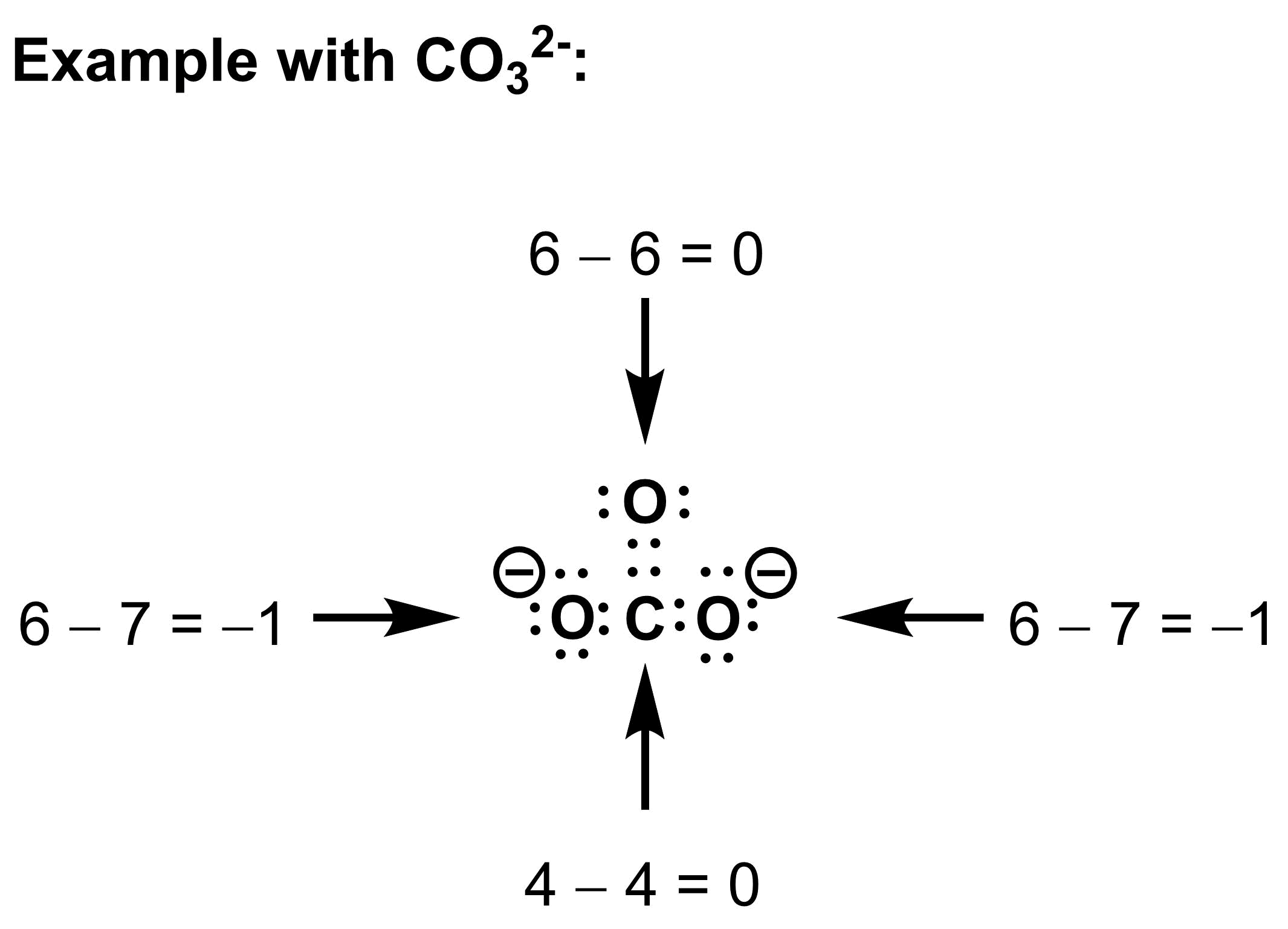
To construct a Lewis dot structure, we must determine the number of valence electrons we have to work with, then arrange them to give as many atoms as possible a full valence shell. As an example, let’s construct a Lewis dot structure for the carbonate anion, CO32-:
1. Determine the number of valence electrons
The total number of valence electrons depends both on the atoms and the charge of the molecule.
a) The number of valence electrons that each atom contributes depends on its placement in the periodic table. If an atom appears in the nth column from the left (ignoring the transition metals), then it will contribute n valence electrons.
b) In a neutral molecule, the total number of valence electrons is simply the sum of the valence electron contributions of each atom from part a. If a molecule is negatively charged, add one electron to the total for each negative charge. If a molecule is positively charged, subtract one electron from the total for each positive charge.
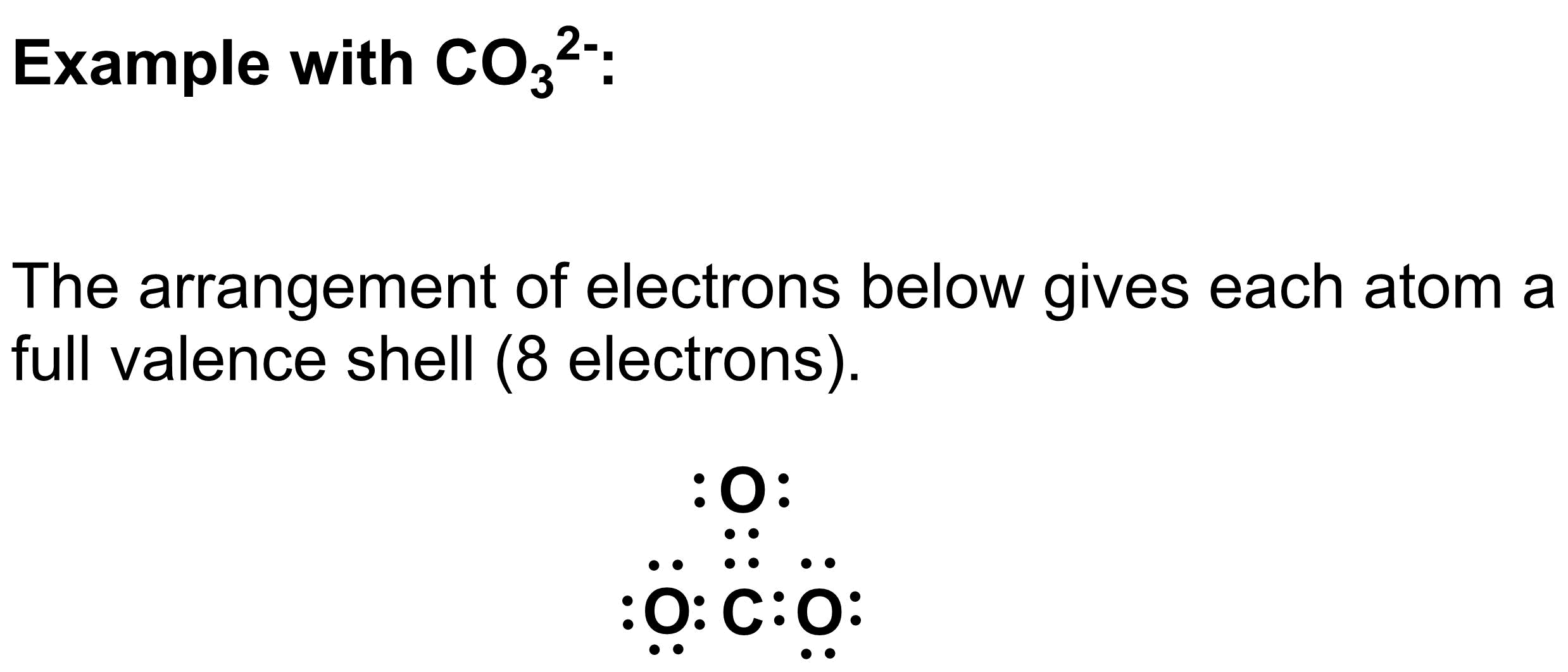
2. Arrange the electrons
Draw the electrons as dots. Arrange the electrons as either lone pairs (isolated on single atoms) or bonding electrons (drawn between atoms) to give each atom a full valence shell. For most atoms in organic molecules, a full valence shell will have 8 electrons (octet rule). For hydrogen, a full valence shell has 2 electrons (duet rule).
3. Assign formal charges
Calculate the formal charge of each atom using:
Formal charge = number of valence electrons – number of associated electrons.
When determining the number of associated electrons, include all lone pair electrons, but only half of the bonding electrons for each atom.
What if multiple Lewis dot structures are possible?
In the example with CO32-, we drew the negative formal charges on the oxygens on the left and the right. But what if we had drawn one of the alternate structures below, instead?

These two structures also each have two negative formal charges on oxygen atoms. All three structures are equally valid Lewis dot structures of CO32-.
Sometimes it is possible to draw more than one valid Lewis dot structure, but the structures aren’t equivalent. In this case, the best (i.e. most stable) one has the most atoms with a full valence shell, the most covalent bonds and the fewest formal charges. If formal charges are present, negative charges should be on the most electronegative atoms and positive charges should be on the least electronegative atoms. For example, if you were asked to draw the Lewis dot structure for H2CCHO- there are two reasonable possibilities, shown below:

While both of these are valid Lewis dot structures, the structure on the left is better than the structure on the right because the negative charge is on the more electronegative oxygen atom.
Interactive:
View solution (try yourself before checking!):