- Isomers are different molecules with the same molecular formula
- Constitutional isomers have the same molecular formula but the atoms have different connectivity
In this section, you’ve seen some of the ways that chemists represent organic molecules. Using these representations, we are able to easily depict a particular molecule. For example, the molecular formula C4H7FCl2 could represent many different isomers, or compounds that share the same molecular formula. Using any of the representations we’ve studied, we could specify a single one of these structures. For example, the four wedge-dash structures below all have the same molecular formula, C4H7FCl2, but we can see that their atoms are connected in different ways, so they represent different molecules. These molecules are constitutional isomers of each other.

The structures above are all drawn in a similar way, so it is relatively straight-forward to see that they are different. But what can we do if molecules are depicted using different representations or mixtures of representations? The four structures below all have the same molecular formula, but how can we tell if they are constitutional isomers or different depictions of the same molecule?
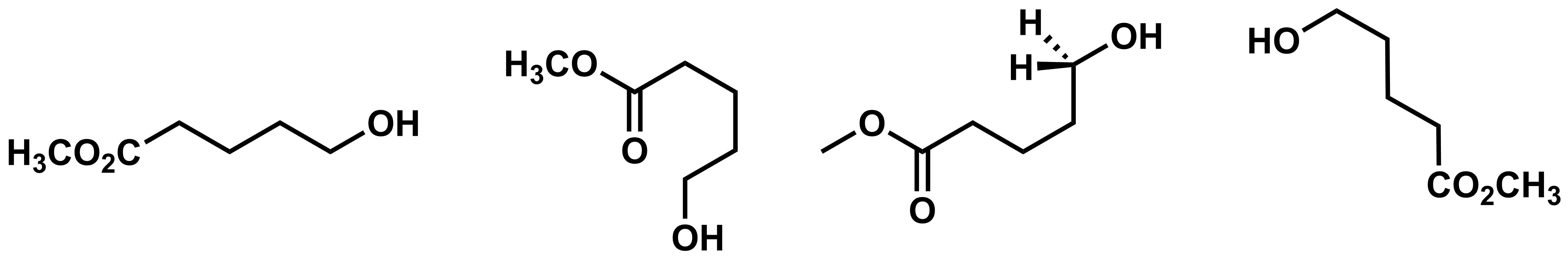
One way to determine if two structures are identical is by comparing their names. If two structures have the same name, then they are the same molecule. All four of the structures above have the name, methyl 5-hydroxypent-2-enoate, so they must all represent the same molecule. In the next section you’ll learn how to name organic molecules like these.
The structures above made use of some common condensed structure abbreviations. To learn more about these common abbreviations, expand the section below.
Common abbreviations in condensed structures:

Interactive: