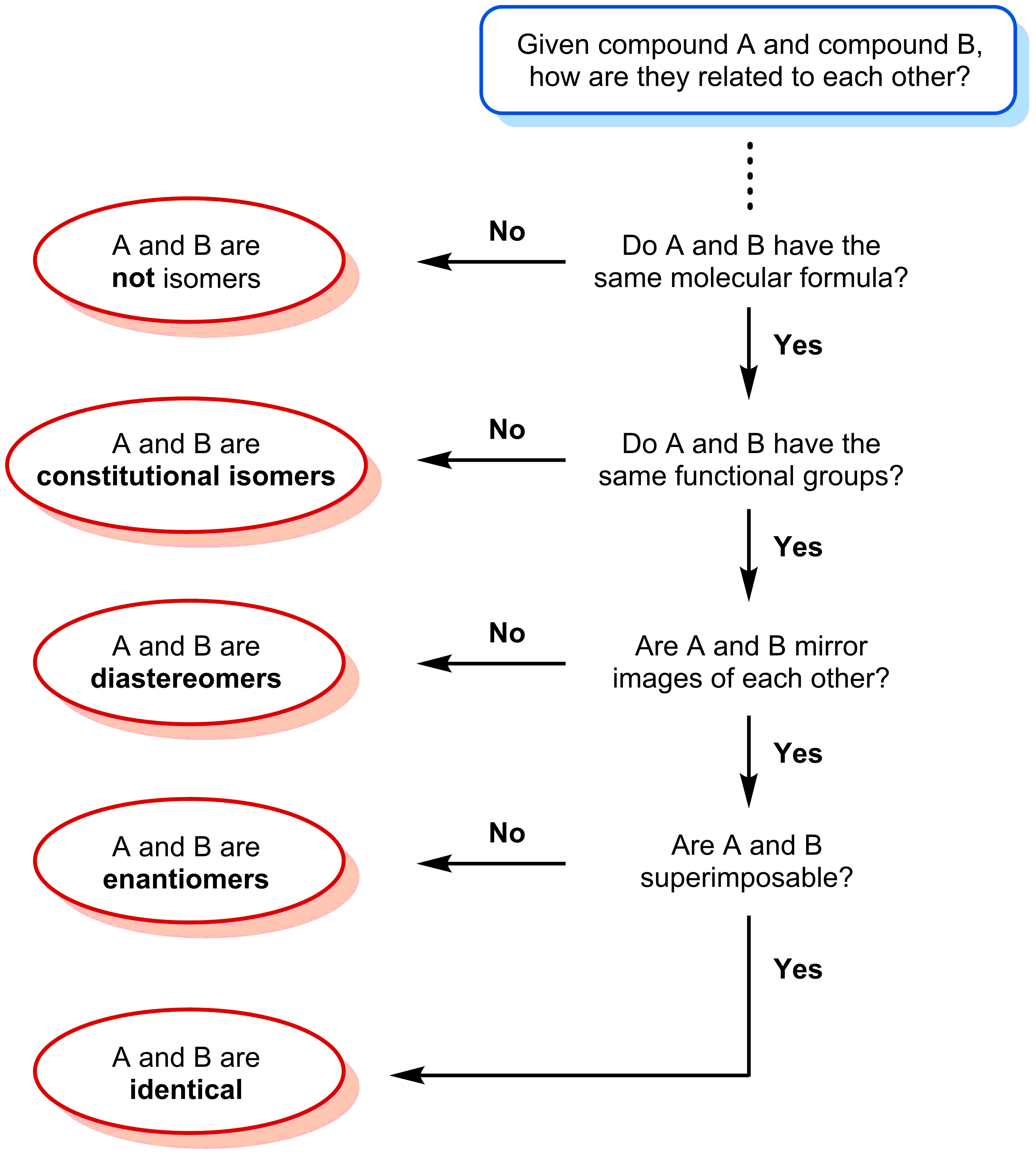
- Constitutional isomers have the same molecular formula, but different connectivities.
- Stereoisomers have the same connectivity, but different arrangements of their atoms in 3D space.
- Enantiomers are stereoisomers that are mirror images of each other.
- Diasteriomers are stereoisomers that are not mirror images of each other.
Lets have a quick review on the different types of isomers you have seen up to this point:
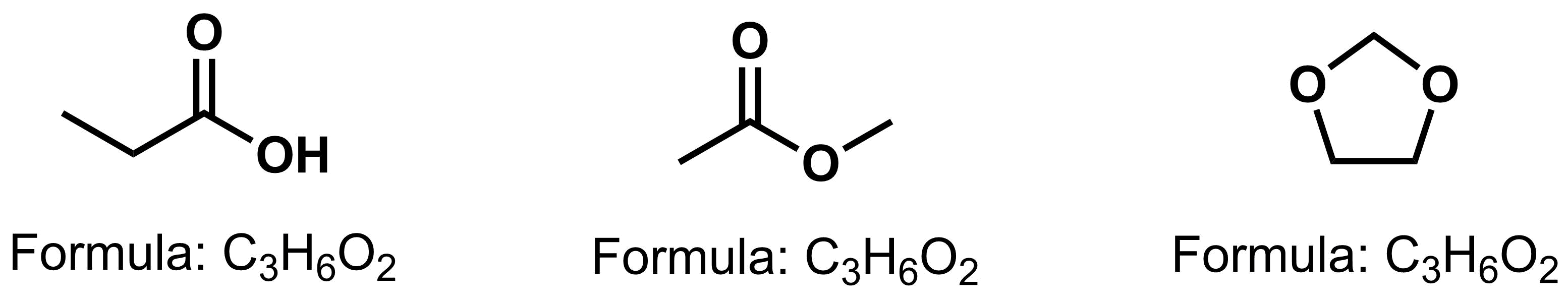
- Constitutional isomers - compounds that share the same molecular formula, but differ in connectivity. The three compounds below all have the same molecular formula of C3H6O2, yet each of the compounds have a different functional group.
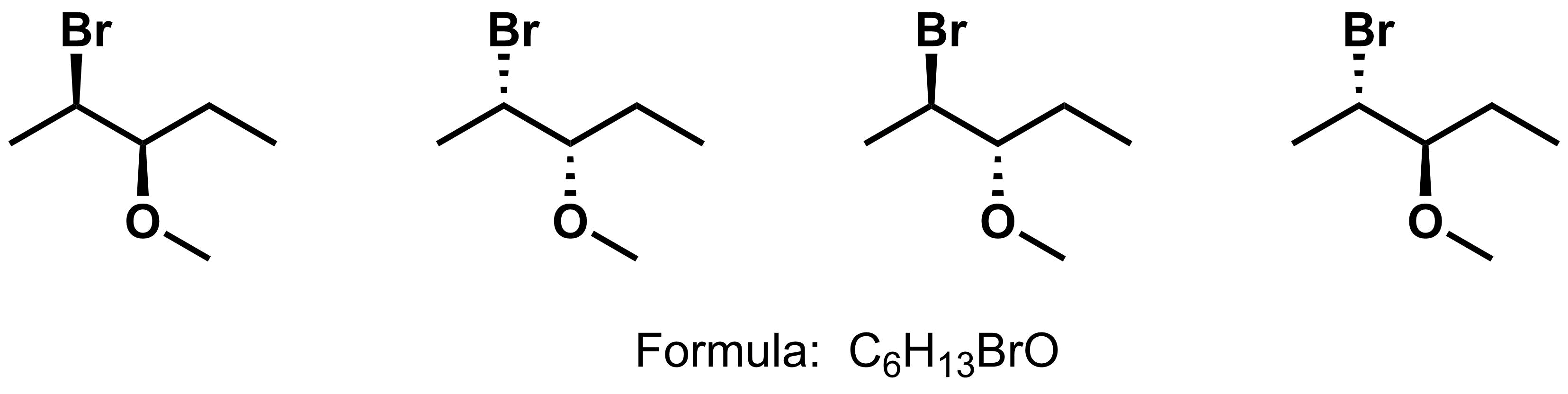
- Stereoisomers - compounds that have the same molecular formula and same connectivity. The four compounds below have the same molecular formula of C6H13BrO and same functional group. The only difference is the spatial arrangements at the asymmetric centers.
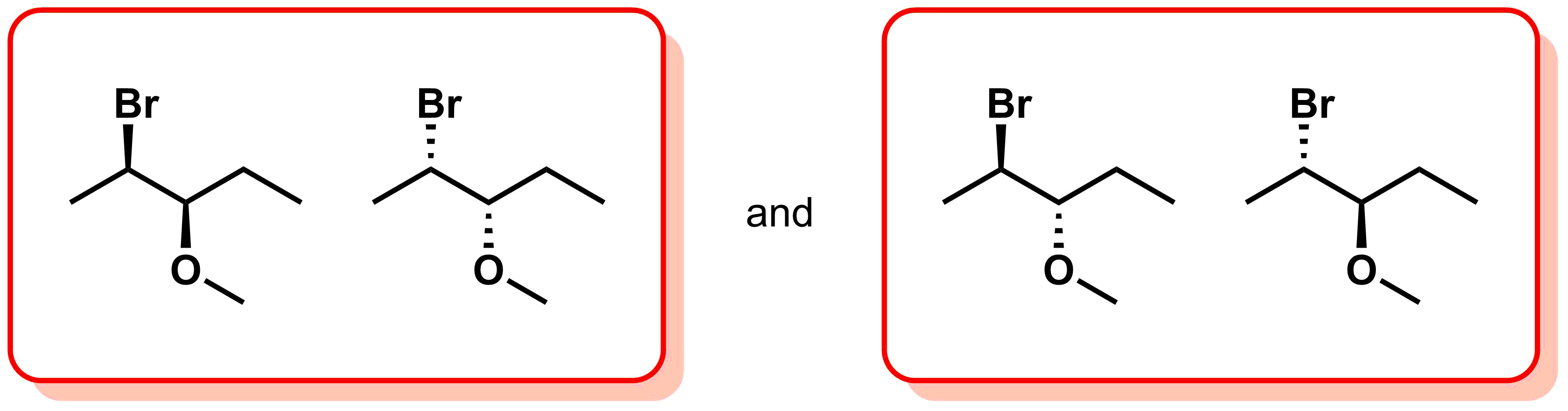
There are two types of stereoisomers: enantiomers and diastereomers.
- Enantiomers - stereoisomers that are non-superimposable mirror images of each other.
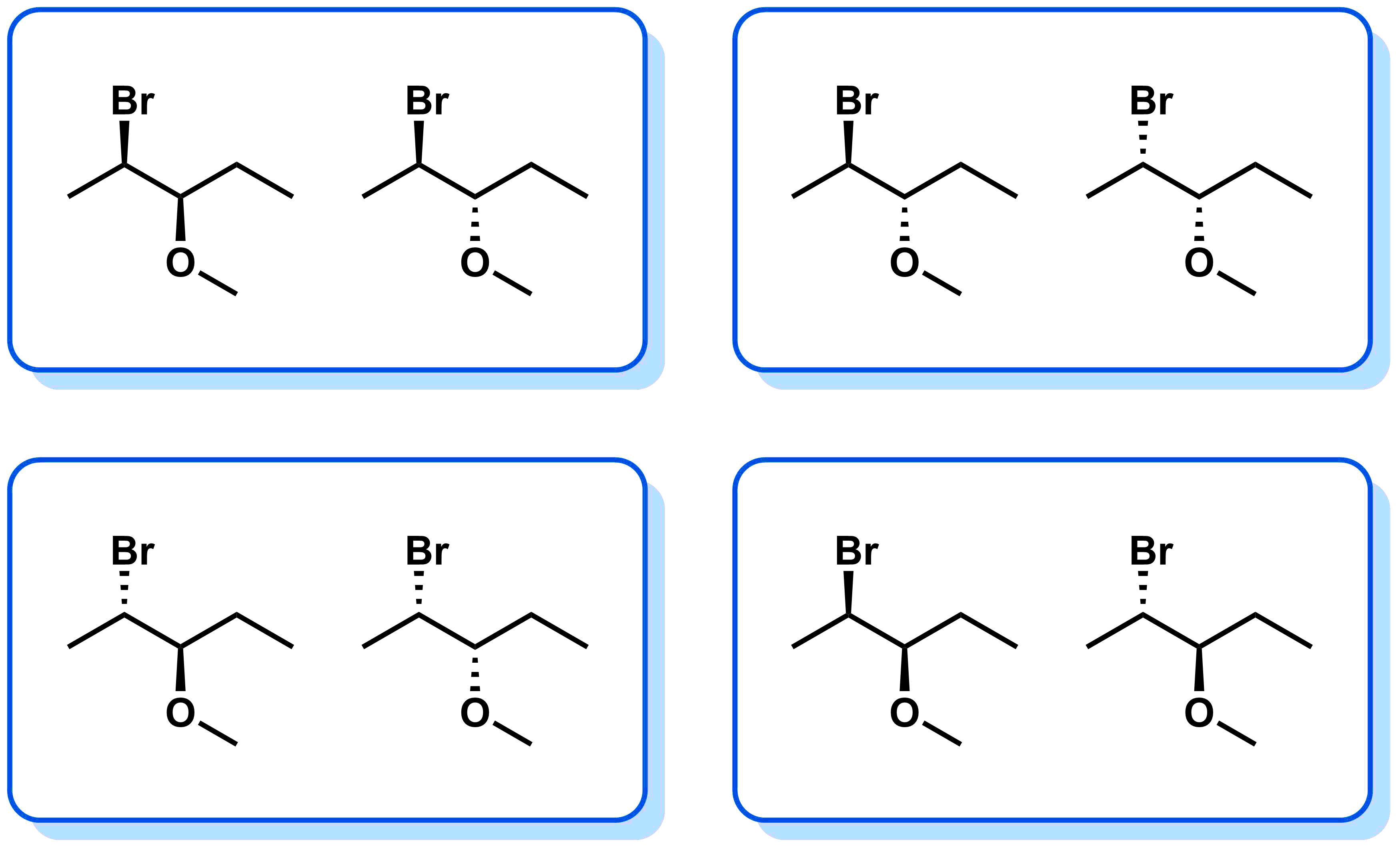
- Diastereomers - stereoisomers that are not enantiomers
The chart below summarizes the process to determine which type of isomer a pair of compound belongs to.
Interactive:
Interactive (Challenge questions):