- In acid-base reactions, the more stable species are favoured at equilibrium
- If an acid-base reaction has a charged species on each side, then we can use electronegativity, induction, polarizability, and resonance to predict which side is more stable
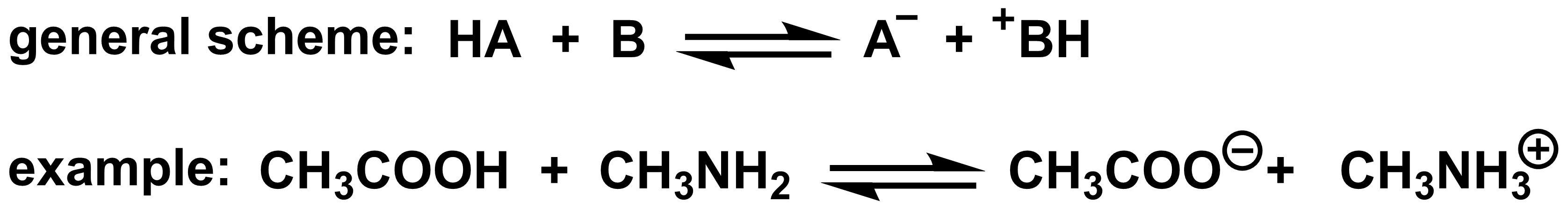
In a Brønsted-Lowry acid-base reaction, an H+ is transferred from an acid to a base. We can group these reactions into three types depending on whether the starting acid and base are neutral or charged.
- A neutral acid (HA) transfers a H+ to a neutral base (B) to give an anionic conjugate base (A-) and cationic conjugate acid (BH+).
- A neutral acid (HA) transfers an H+ to an anionic base (B-) to give an anionic conjugate base (A-) and a neutral conjugate acid (BH).

- A cationic acid (HA+) transfers an H+ to a neutral base (B) to give a neutral conjugate base (A) and a cationic conjugate acid (BH+).

Acid-base reactions are thermodynamically controlled. That is, the energy of activation of an acid-base reaction is generally small so that most molecules have sufficient energy to overcome this barrier and thus equilibrium is established. The position of the equilibrium depends on the relative stabilities (i.e. Gibbs free energies) of the reactants and products. The side with the more stable molecules will be more favoured in the equilibrium. We’re most interested in the relative stabilities of charged species, since these are usually the least stable molecules in the reaction so they will have a larger effect on the overall stability. In Type 1 acid-base reactions (neutral reactants to charged products), the two charged species are both on the same side of the equilibrium, so we cannot use their relative stabilities to help us predict the position of the equilibrium (we’d need to know pKa data to make a prediction). However, in both Type 2 and Type 3 reactions, there is a single charged molecule on each side of the equilibrium. The equilibrium will favour whichever side has the more stable charged molecule.

We can determine which charged species is more stable by comparing their relative electronegativity, induction, polarizability and resonance. Molecules are generally more stable if formal charges (both positive and negative) are distributed throughout the bonding network.
Quick review on determining relative stabilities of charged species
There are two general cases that arise that allow us to predict the relative stabilities of two charged species:
- In each charged molecule the formal charge is isolated on a single atom and these atoms are different elements.
- If the elements are in the same row of the periodic table, compare their electronegativities.
- More electronegative atoms are better at stabilizing negative charges
- Less electronegative atoms are better at stabilizing positive charges - If the elements are in the same column of the periodic table, compare their polarizabilities.
- More polarizable (bigger) atoms are better at stabilizing negative charges - The atoms that bear the formal charge in both charged molecules are the same element.
- Check for resonance
- If one charged species has more good resonance structures than the other, it is more stable - Check for induction
- Electronegative atoms near a negative formal charge stabilize the molecule through induction
- Electronegative atoms near a positive formal charge destabilize the molecule through induction - Compare the atoms’ hybridizations
- Atoms with hybridized orbitals that have more s-character are more electronegative
Interactive: