- CIP rules systematically prioritize each substituent, where higher atomic number corresponds to higher priority.
When a molecule contains a double bond, there are often two different possible isomers. To unambiguously name such molecules, we need a system of nomenclature that indicates which isomer of the double bond is present. To differentiate between the two possible isomers, we use the Cahn-Ingold-Prelog (CIP) rules. We'll use these same rules again to differentiate between isomers in the next section.
The first step to naming substituted alkenes is to assign a priority to the two substituents attached to each side of an alkene. Higher priority substituents will be ranked “high” and the lower priority substituent will be ranked “low”. Please remember to always compare the same carbon atom in the double bond, not substituents across the double bond.
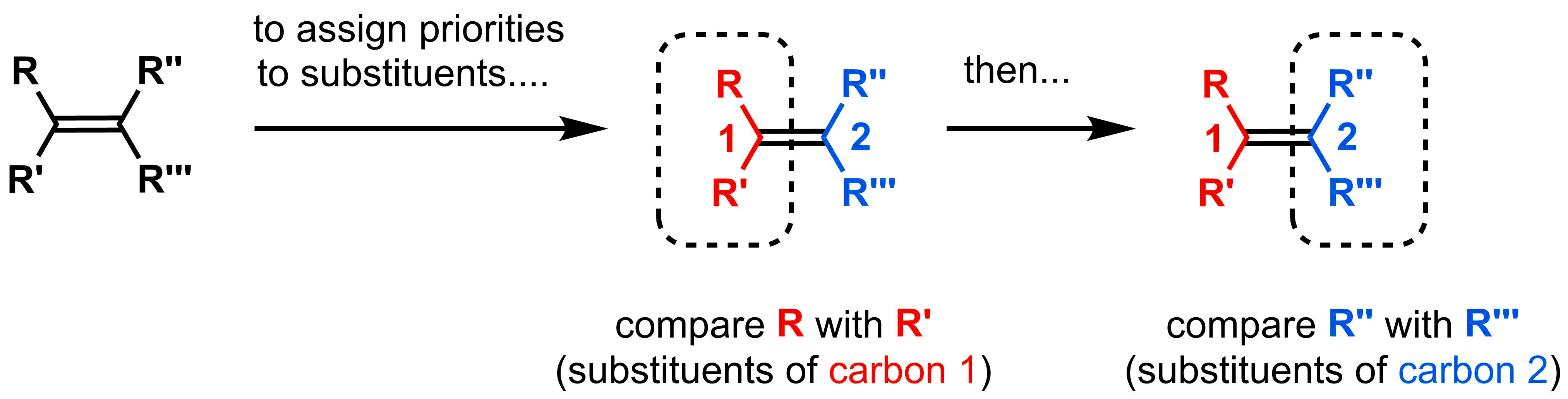
To decide which substituent is "high" and which is "low", we will use a set of guidelines called the CIP rules, which are summarized below
- 1. Atoms with higher atomic numbers have higher priority.
- 2. If rule 1 results in a tie, consider all atoms 2 bonds away. Repeat this process until a priority can be decided.
- 3. Count an n-multiple bond as n single bonds.
Below, each of the rules will be applied to specific examples.
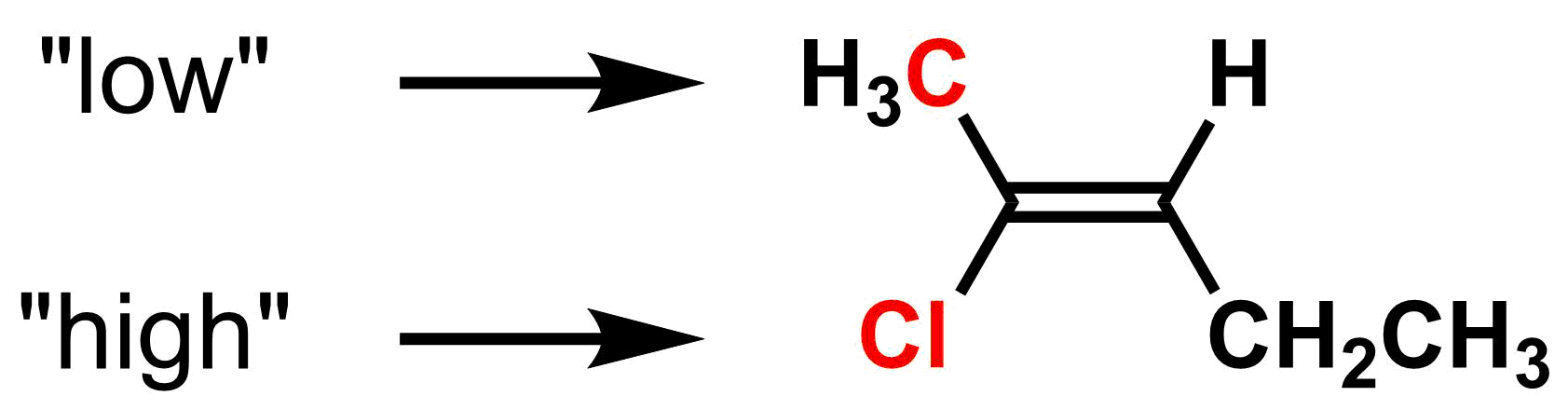
Rule 1: Atoms with higher atomic numbers have higher priority.
Compare the atoms directly attached to only one side of the alkene. Whichever has the higher atomic number has the higher priority. For example, on the left side of the molecule below, Cl (atomic number 17) has higher priority than carbon (atomic number 6). Thus, the methyl group is lower priority than the chlorine.
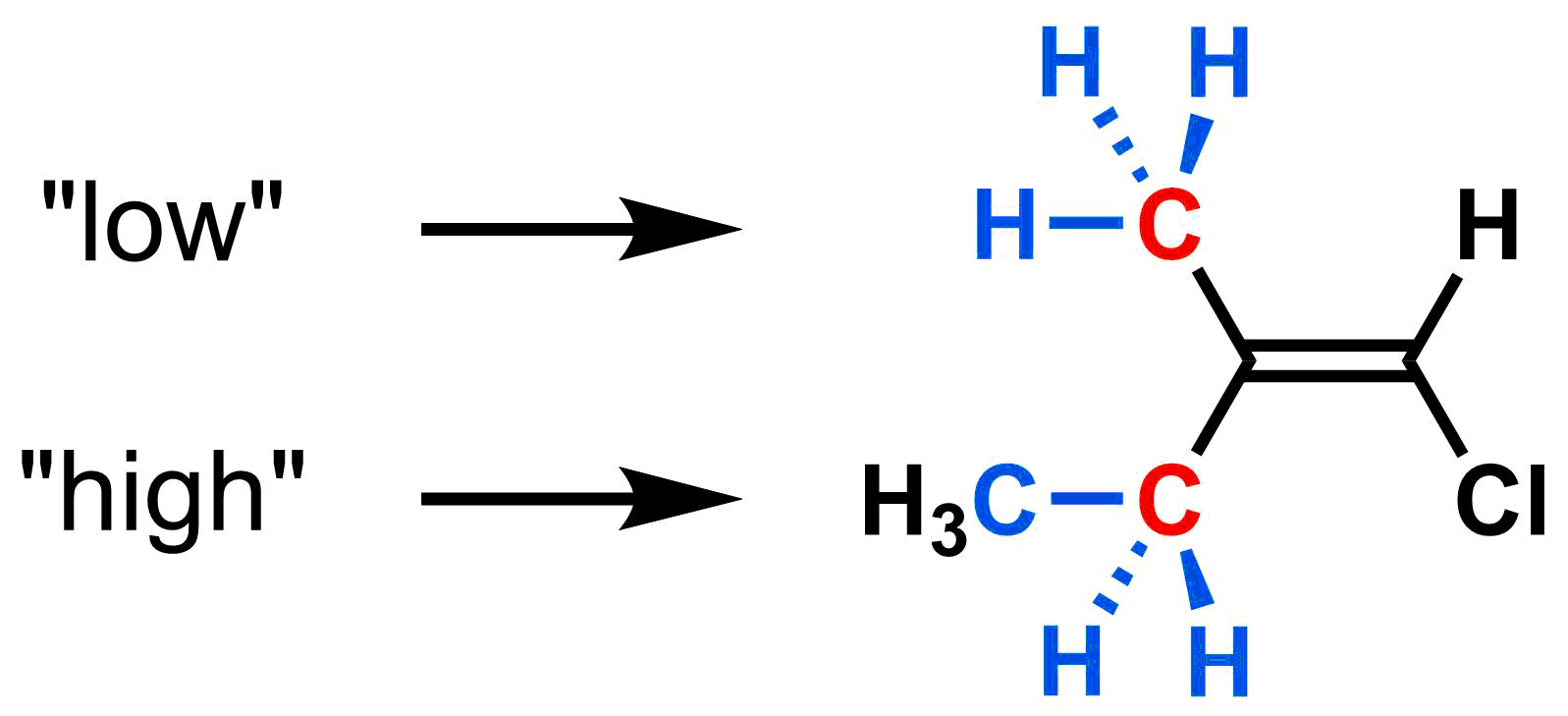
Rule 2: If rule 1 results in a tie, consider all atoms 2 bonds away. Repeat this process until a priority can be decided.
If rule #1 results in a tie, consider all atoms in a substituent that are 2 bonds away from the alkene carbon. If there is still a tie, continue to look further away, one bond at a time (following all branches), until there is a difference. For example, consider the left side of the alkene below. The top and bottom substituents both have a C (shown in red) directly bonded to the alkene carbon. Since there is a tie, we must consider all atoms two bonds away from the alkene carbon. The top substituent has H,H,H (shown in blue) two bonds away. The bottom substituent has C,H,H (shown in blue) two bonds away. Since the bottom group has the highest atomic number (C's atomic number is 6, while' H's is 1), it has the higher priority.
3. Count an n-multiple bond as n single bonds.
If your molecule contains a multiple bond, when determining the CIP priority consider each π-bond as two single imaginary bonds (one from each atom in the multiple bond). As a result, double bonds count as two single bonds, and triple bonds count as three single bonds. For example, consider the C=O below. Keep the C-O σ-bond, but count the C as having an extra "imaginary bond" to O and O as having an extra "imaginary bond" to C.
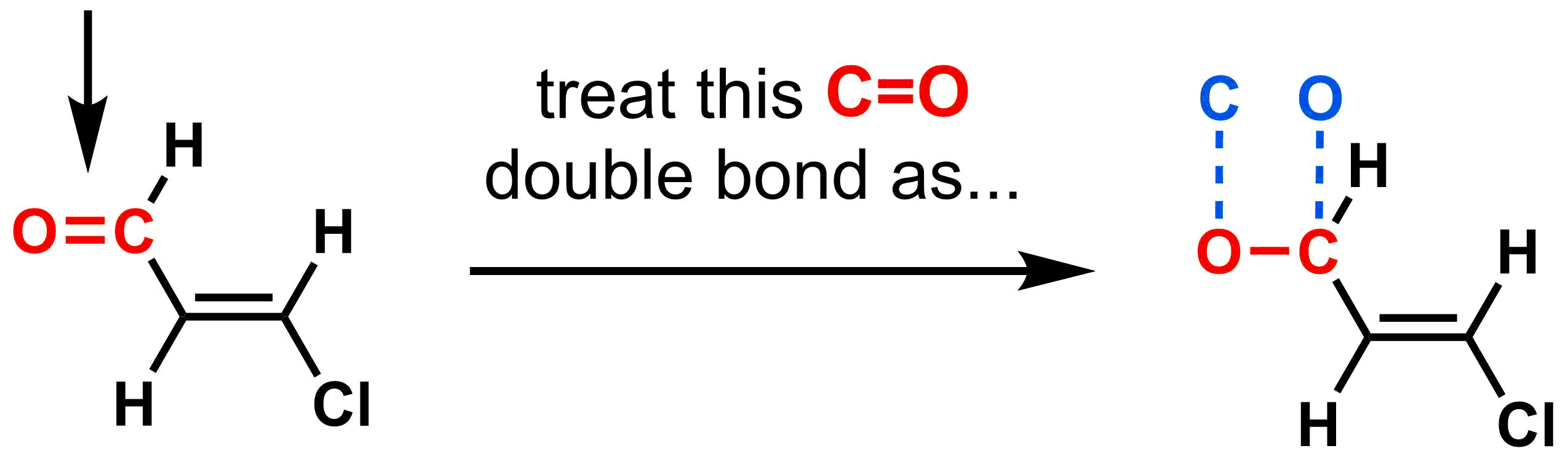
Interactive: