- Constitutional isomers have the same molecular formula, but different connectivity.
- Stereoisomers have the same connectivity, but different spatial arrangements.
- Enantiomers and diastereomers are two types of stereoisomers.
As was previously defined in Part 3 (Section 1.6), isomers are two different molecules that have the same molecular formula. Isomers can be further subcategorized based on how the molecules differ. For example, if the two molecules have the same molecular formula but have different connectivity, they are defined as constitutional isomers. This section will focus on a different category, stereoisomers, which have the same molecular formula and the same connectivity, but different in the arrangement of the atoms in space. Note that conformers are not stereoisomers as the former can interconvert through bond rotations while the latter cannot.
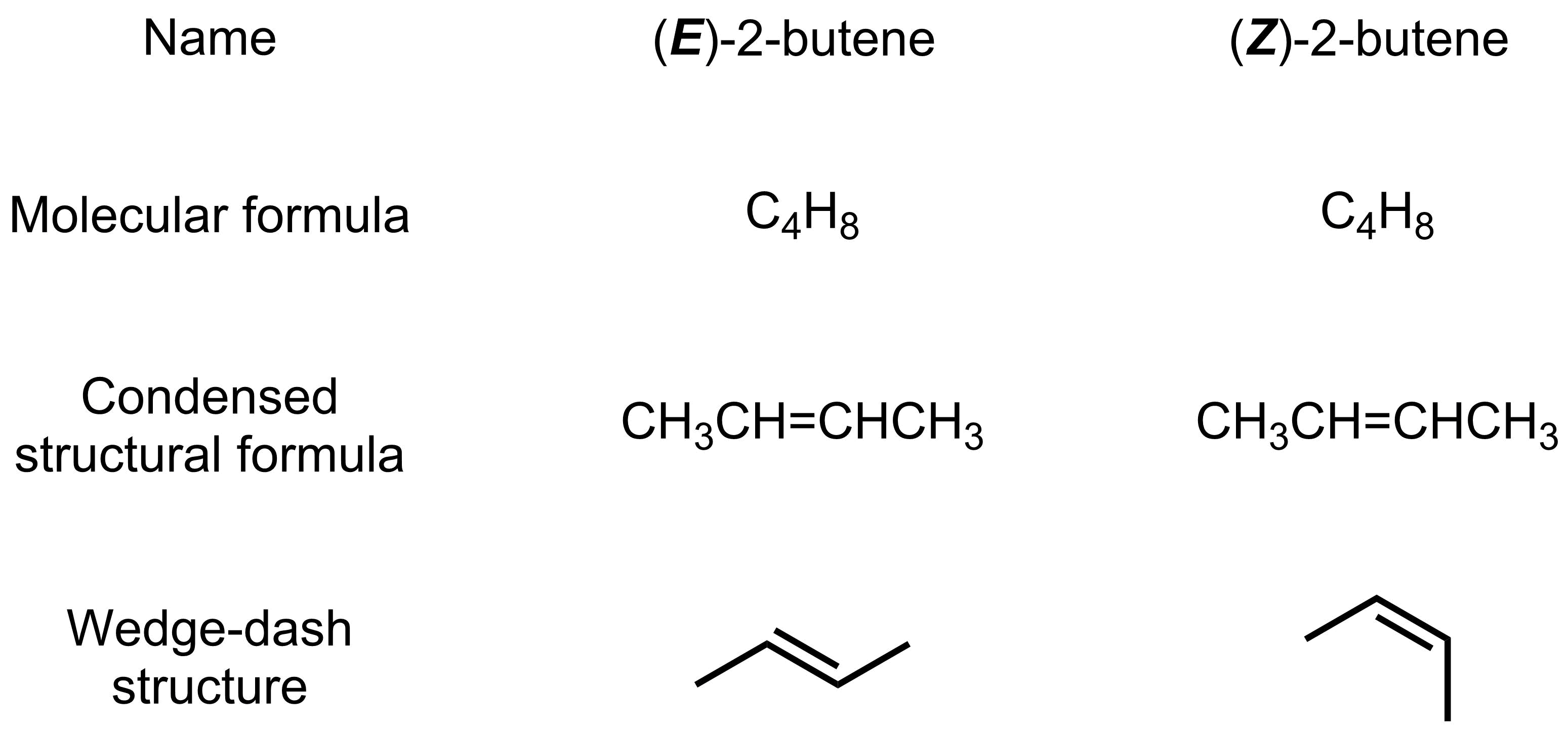
An example of stereoisomers that you have already seen is (E)- and (Z)-2-butene. These molecules are isomers with the same connectivity (they have the same condensed formula), but the two species are not in equilibrium.
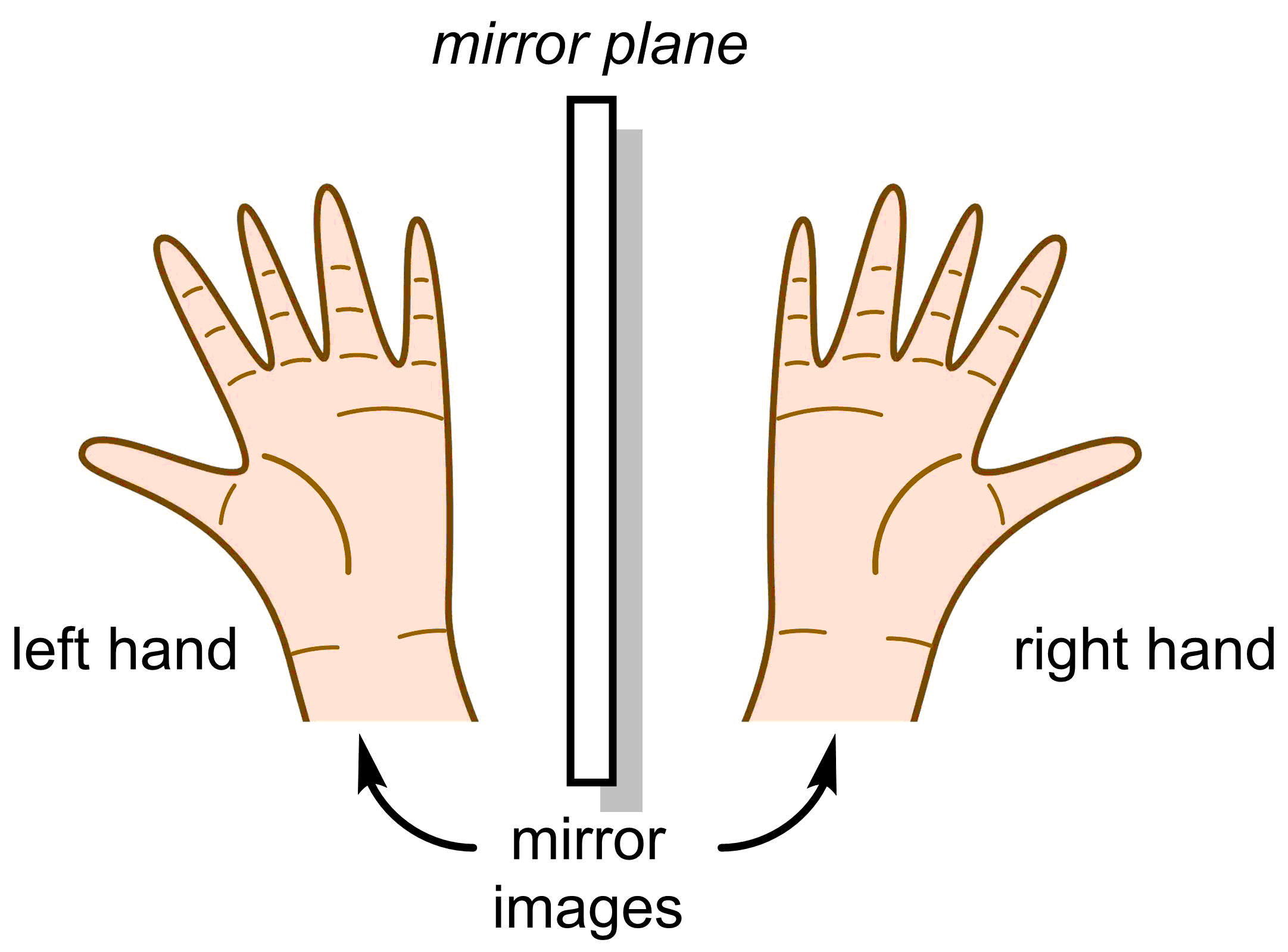
Stereoisomers can be further subdivided into two categories: enantiomers (Sections 2.1-2.2) and diastereomers (Sections 2.3-2.4). Enantiomers are defined as molecules that are non-superimposable mirror images. For example, your hands are enantiomers; they are identical in every way except they are mirror images of each other. They are non-superimposable, meaning that there is no way you can bend your fingers or rotate your left hand to make it identical with the right.
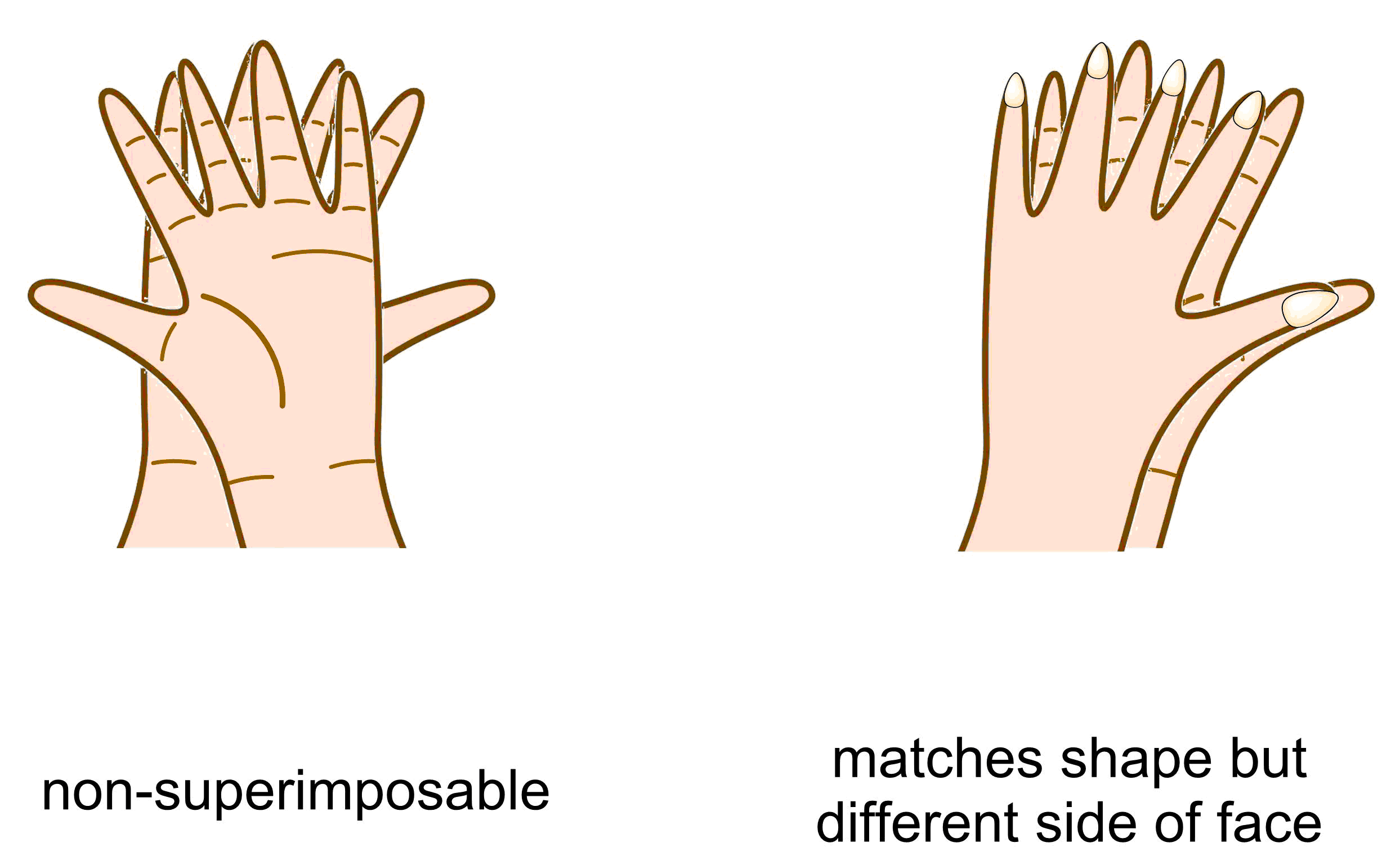
Enantiomers have the important property of being chiral, which is defined as an object, or a molecule, that is non-superimposable with its mirror image. It is important to highlight that chirality is a property while enantiomer is a term used to describe the relationship between two objects. Returning to the hand example, your left hand is chiral (as is your right). The relationship between your left and right hands is they are enantiomers
Interactive: