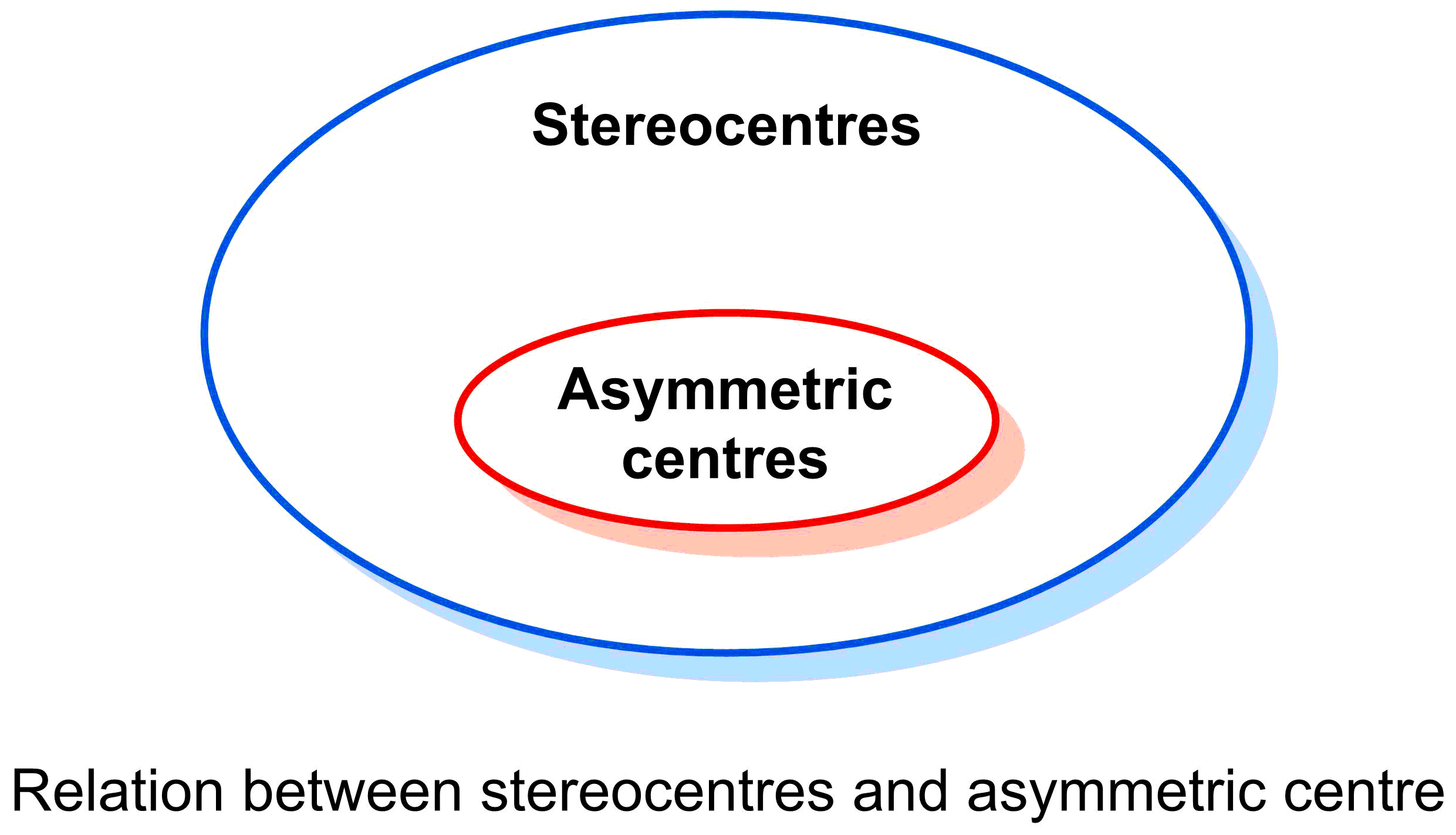
- An atom is a stereocentre if switching the positions of two of its substituents leads to a different stereoisomer.
- An asymmetric centre is a specific type of stereocentre in which an atom has four different substituents.
- The mirror image of a molecule with one asymmetric centre is non-superimposable with (i.e. not identical to) the original molecule.
Chirality at the molecular level is best determined by focusing at the connectivity at certain carbon atoms. One of the first things to look for when analyzing whether a molecule is chiral is to identify asymmetric centres, which are defined as atoms with four different substituents. A generic form of asymmetric centre is shown below, where A, B, C, and D indicates different substituents attached to the carbon atom.
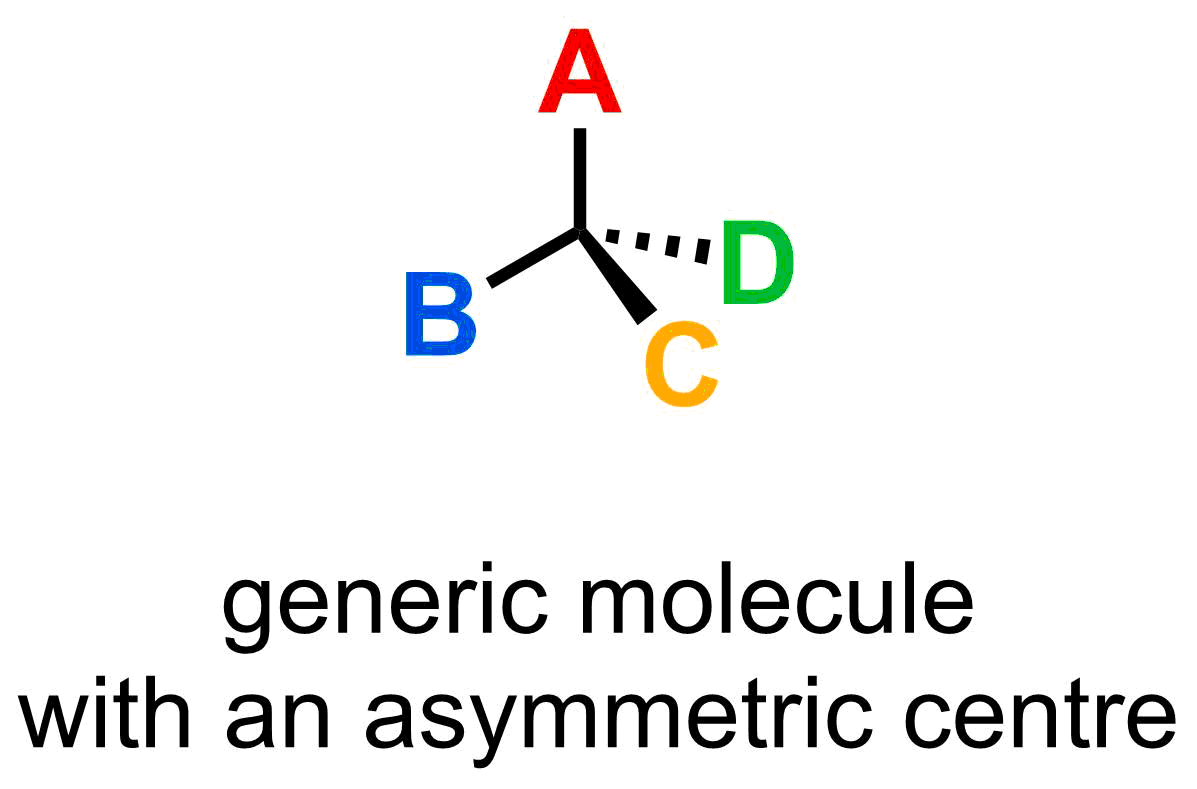
If a molecule has only 1 asymmetric centre, it is always chiral. This can be seen by taking the mirror image of the molecule and comparing the original with the mirror image. As you can see, there is no way to rotate the mirror image to make it look like (i.e. superimpose it with) the original.
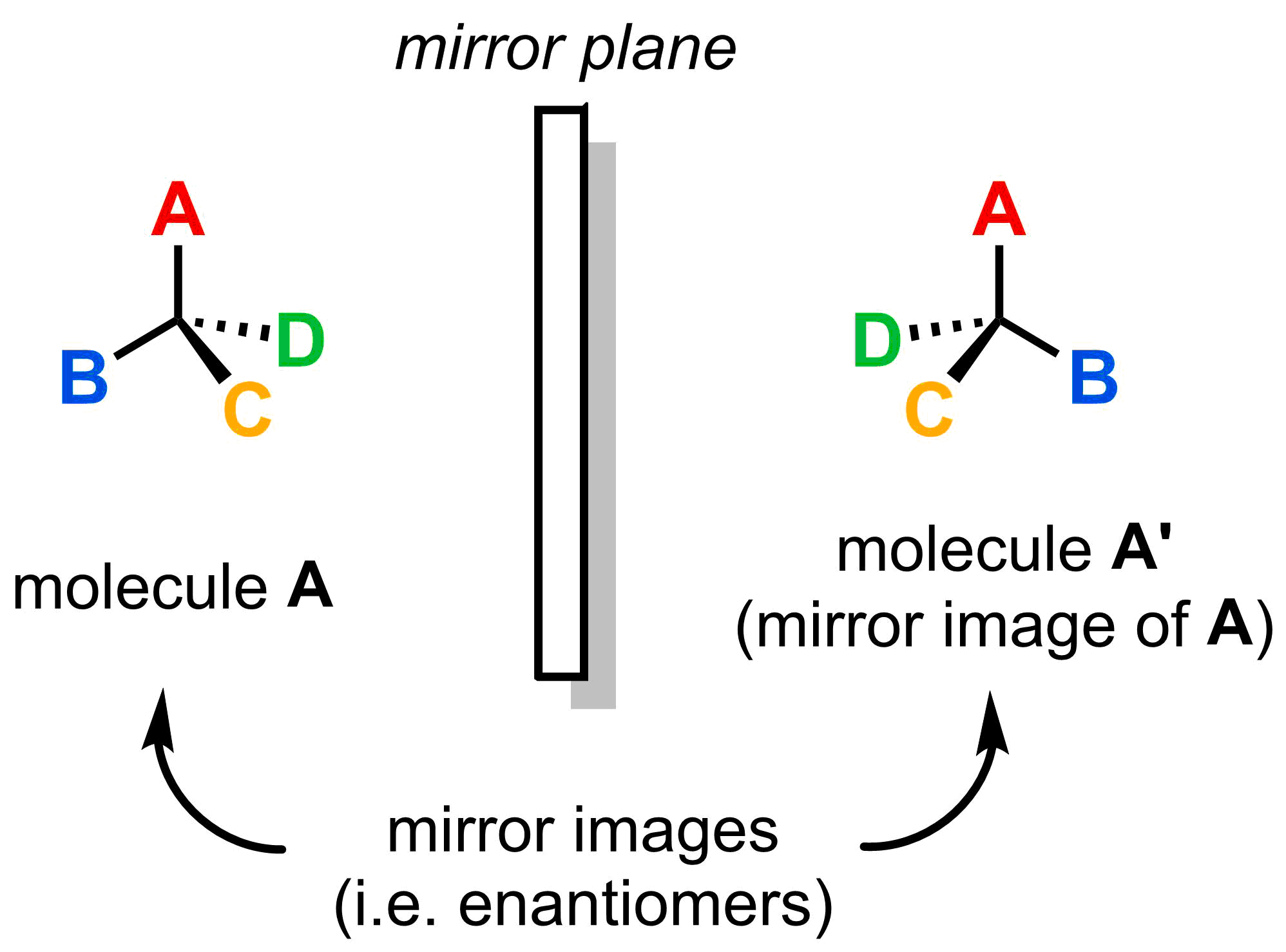
Try rotating the two molecules below to see how the original (molecule A) and its mirror image (molecule A') cannot be superimposed.
| molecule A | molecule A' |
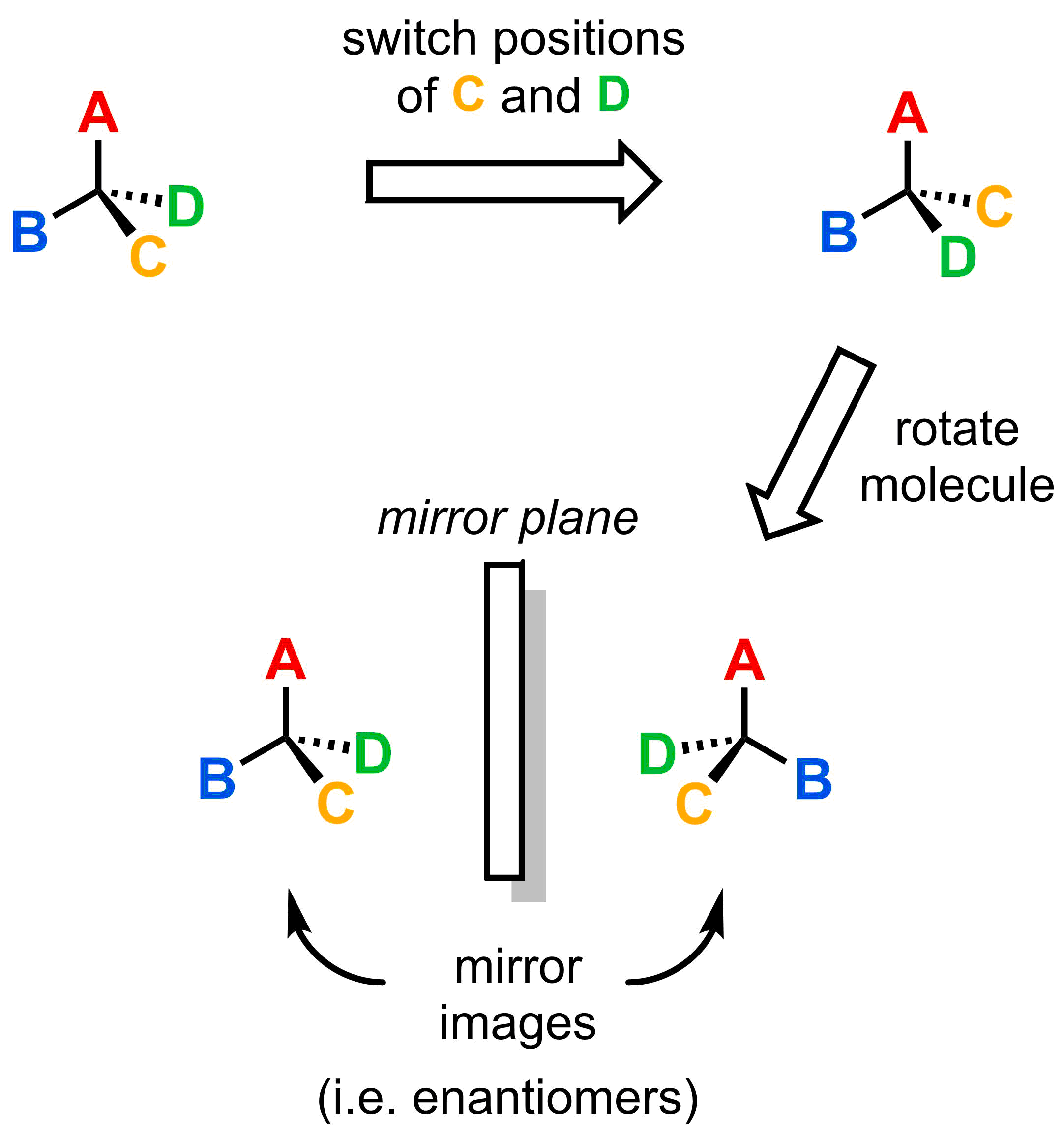
While asymmetric centres are easy to identify, the term is very specific and cannot be broadly applied in future chemistry courses. A more general term is stereocentre. A centre is a stereocentre if switching any two substituent leads to a different stereoisomer. For example, when the position of the substituents C and D for the molecule on the top left has been switched, a new molecule will be generated. The two molecules are not identical with each other but related as a non-superimposable mirror images, and thus are stereoisomers (specifically enantiomers).
Why is a molecule with less than four different substituents not chiral (i.e. a molecule that has no asymmetric centre)? This can be answered in the example below, which only has three different substituents. As you can see, the original molecule (B) and its mirror image (B') are identical (they can be superimposed after rotation), so they cannot be stereoisomers.
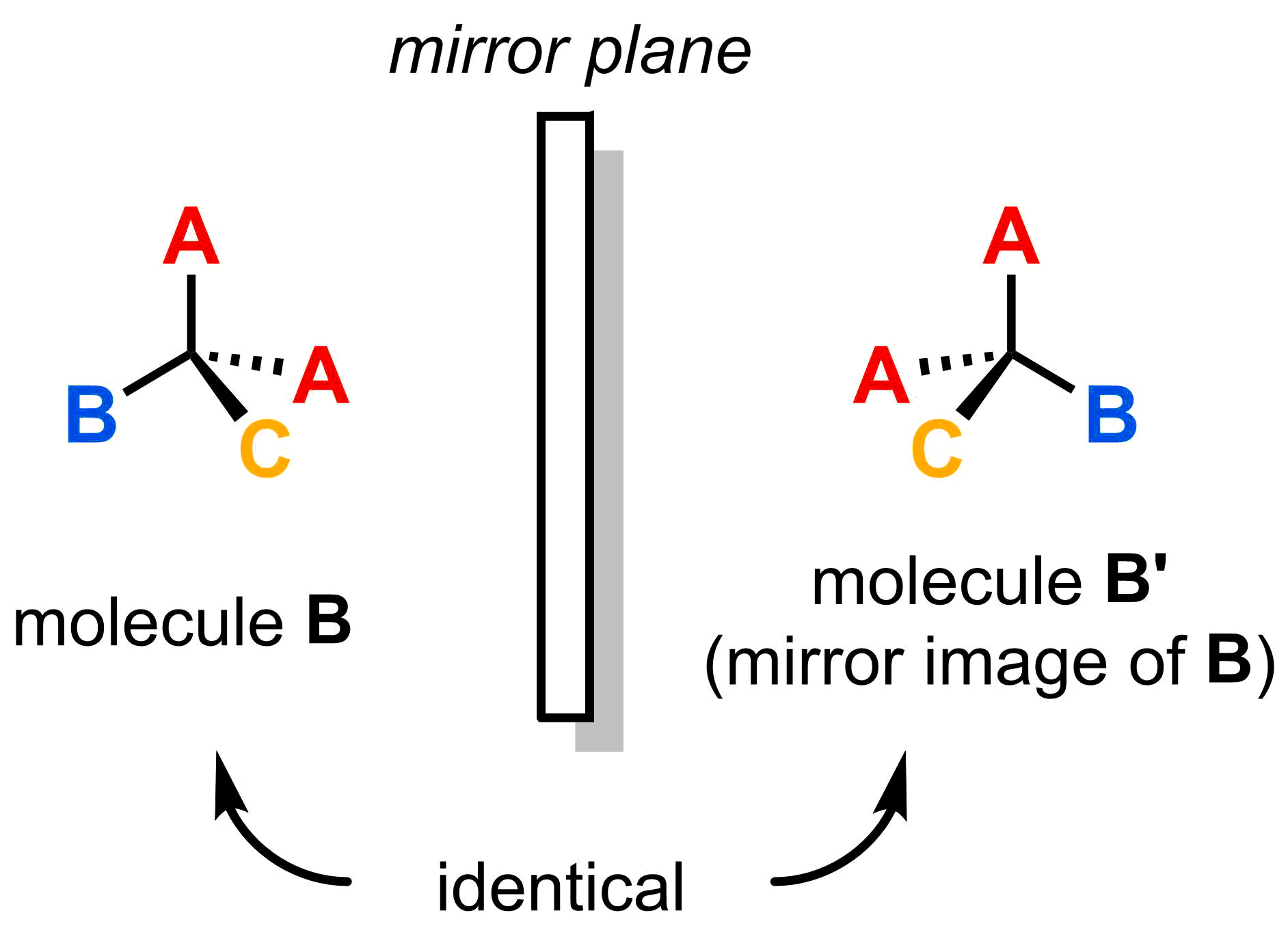
Try rotating the two molecules below to see how the original (molecule B) and its mirror image (molecule B') could be superimposed, hence identical molecules.
| molecule B | molecule B' |
All asymmetric centres are stereocentres, but not all stereocentres are asymmetric centres. For example, the carbons in double bonds that can be labelled with E/Z configuration are stereocentres (switching two substituents on one of these carbon atoms would lead to a different stereoisomer) but not asymmetric centres.
Interactive: