- Enantiomers have identical physical properties.
- Enantiomers can be distinguished from each other only when they interact with other chiral objects.
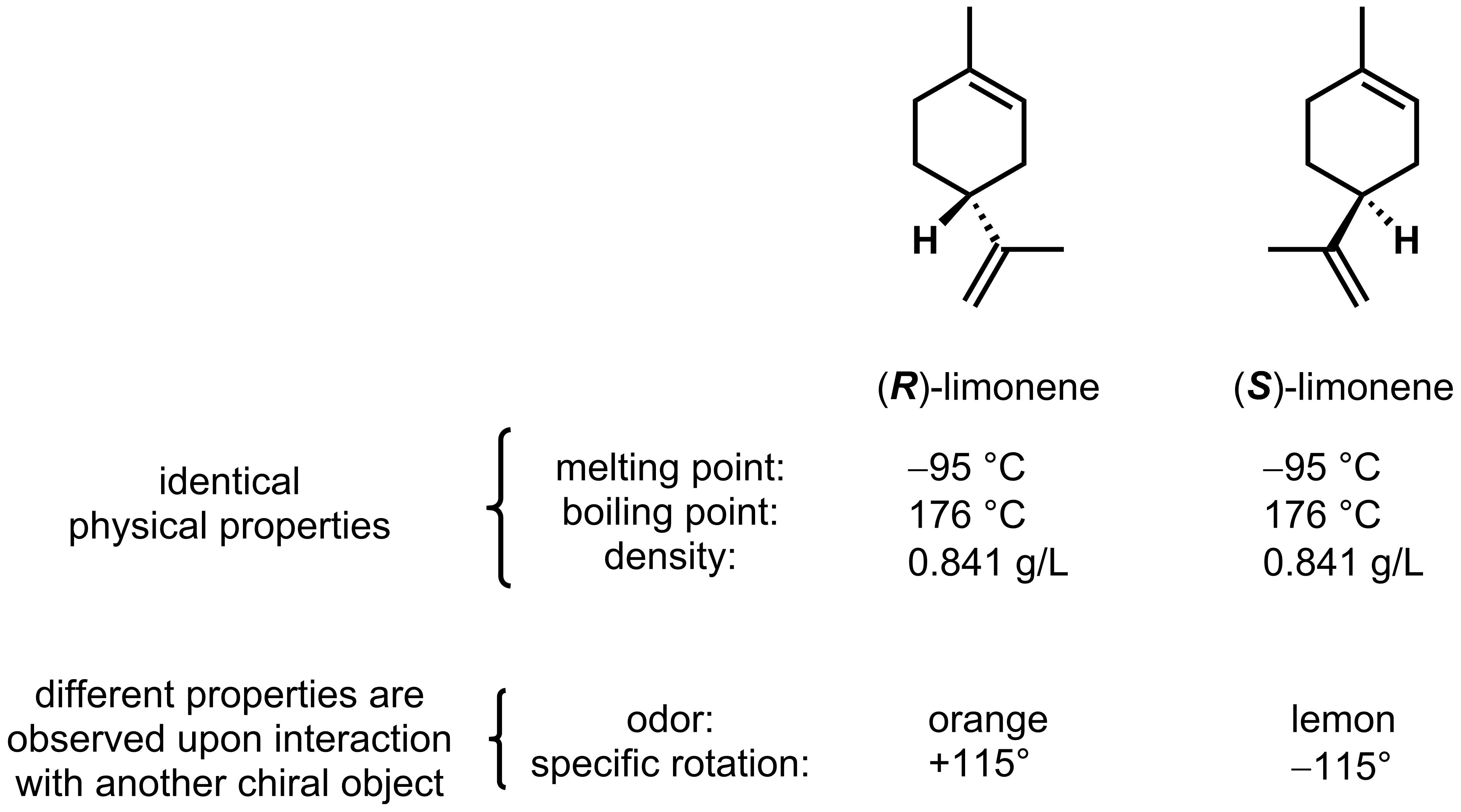
Like constitutional isomers, diastereomers usually have different physical properties. Enantiomers are different from all other categories of isomers in that pairs of enantiomers always have identical physical properties. For example, (R)- and (S)- limonene shown below, have the exact same melting point, boiling point, and density.
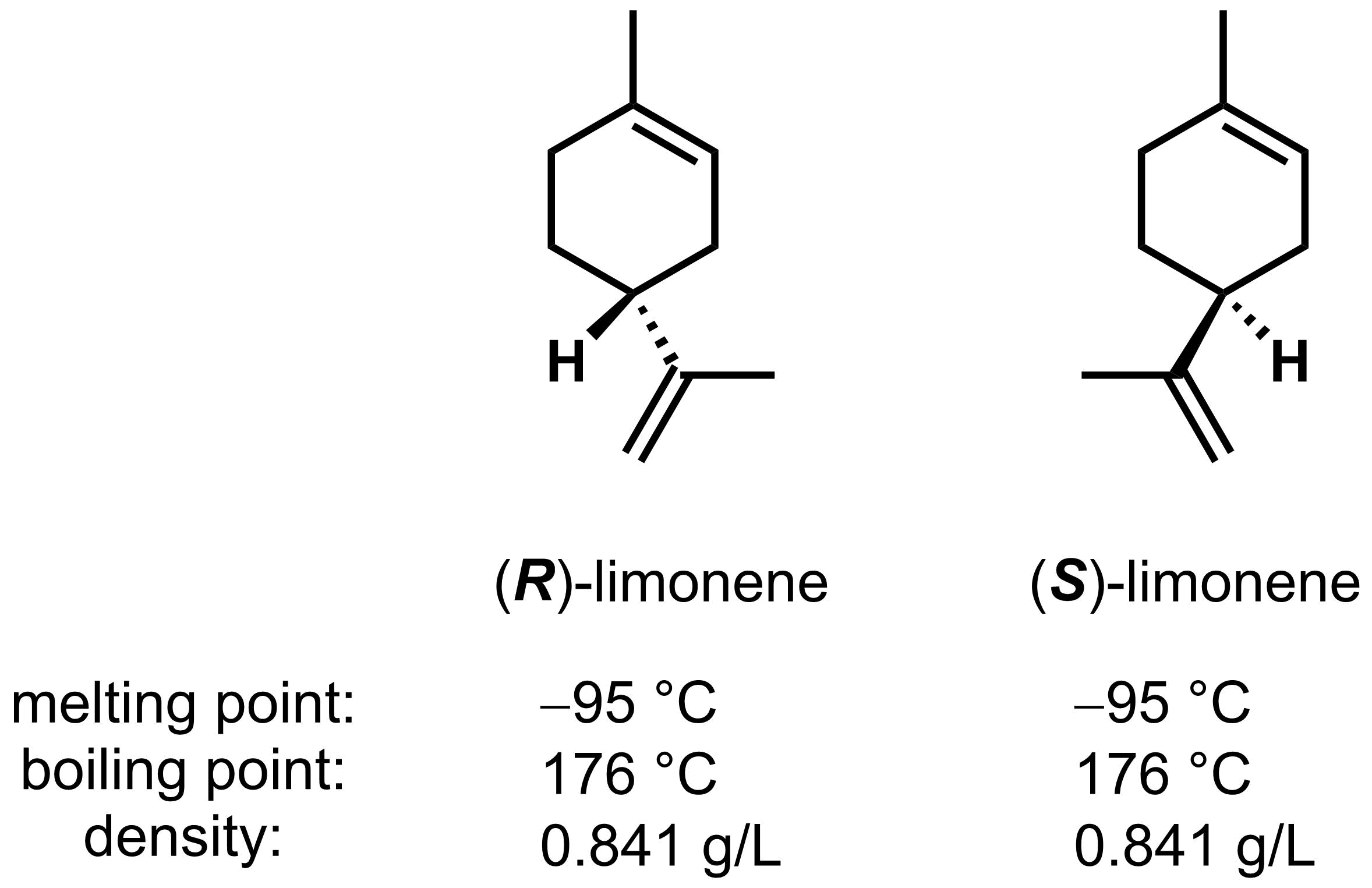
If the physical properties are the same, how can these chiral molecules be differentiated? Chiral molecules exhibit different properties (1) in how they interact with other chiral molecules and (2) how they interact with plane polarized light. Part (1) will be covered in this section, and Part (2) will be covered in Section 2.9.
Interaction of chiral molecules with other chiral species
Chiral molecules can be readily differentiated through their interactions with other chiral species. For example, consider a chiral pharmaceutical drug interacting with a chiral receptor in the body, as shown in the diagram below. Since the (R)-enantiomer on the left can bind with all three sites of the receptor simultaneously, while the (S)-enantiomer cannot, we might expect that the (R)-enantiomer would be a more potent pharmaceutical drug (stronger binding) at this receptor. We could consider these two receptor-molecule complexes as diastereomers of each other (same connectivity but not mirror images), which explains why they have different properties.
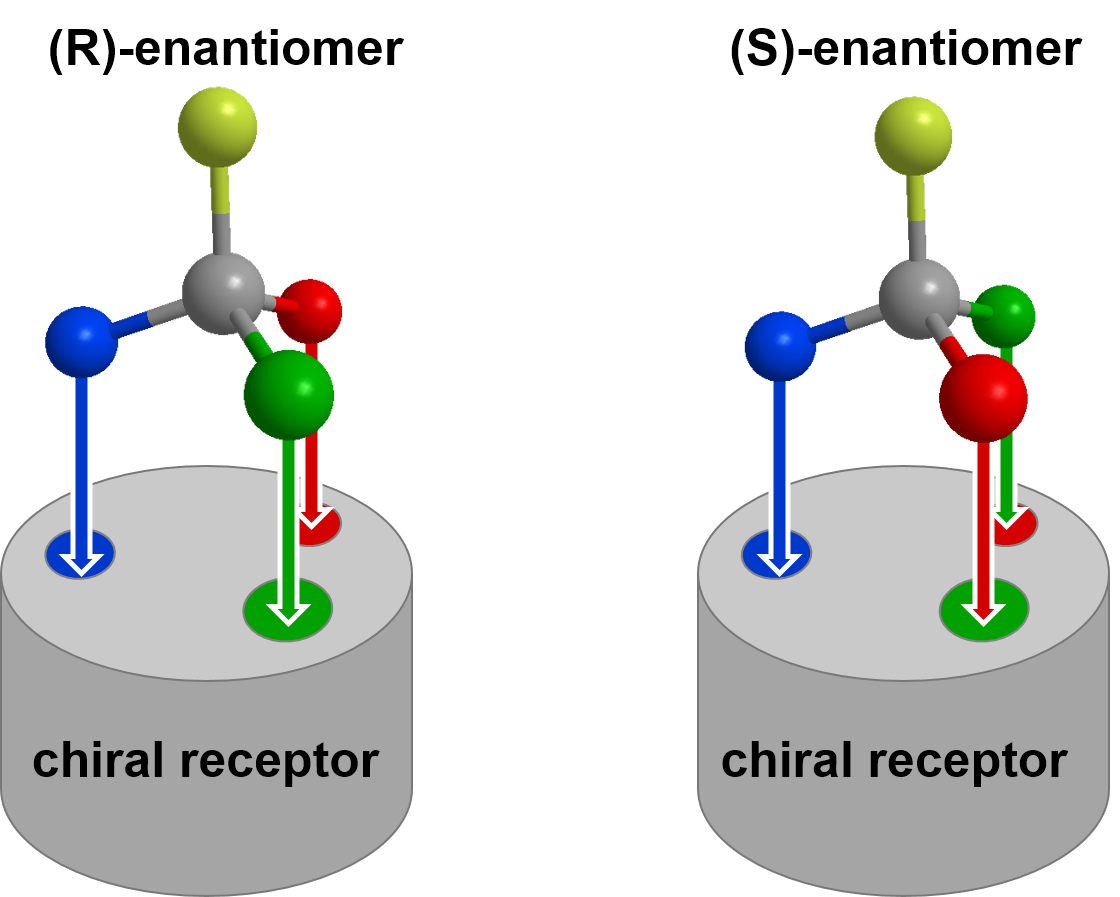
The differing interaction with chiral objects has a very important consequence in biology as all enzyme binding pockets, taste receptors, and smell receptors are chiral. Therefore, chiral objects will often have very different interactions with your body. Let's go back to the limonene example. These two enantiomers can readily be detected by the chiral olfactory receptors in your nose. Each enantiomer binds differently, and in turn you experience a different smell for the two compounds; (R)-limonene smells like oranges and (S)-limonene smells like lemons.
Interactive: