- The heat (q) is the amount of energy that transfers from a higher temperature object to a lower temperature object during a process.
- The work (w) is the amount of energy that transfers due to a force applied over a distance. In Chemistry 123, we focus only on work resulting from expansions and compressions, which is called PV-work.
- The change in a state variable depends only on the initial and final states of a system, while the value of a path variable will differ depending on how this change occurs.
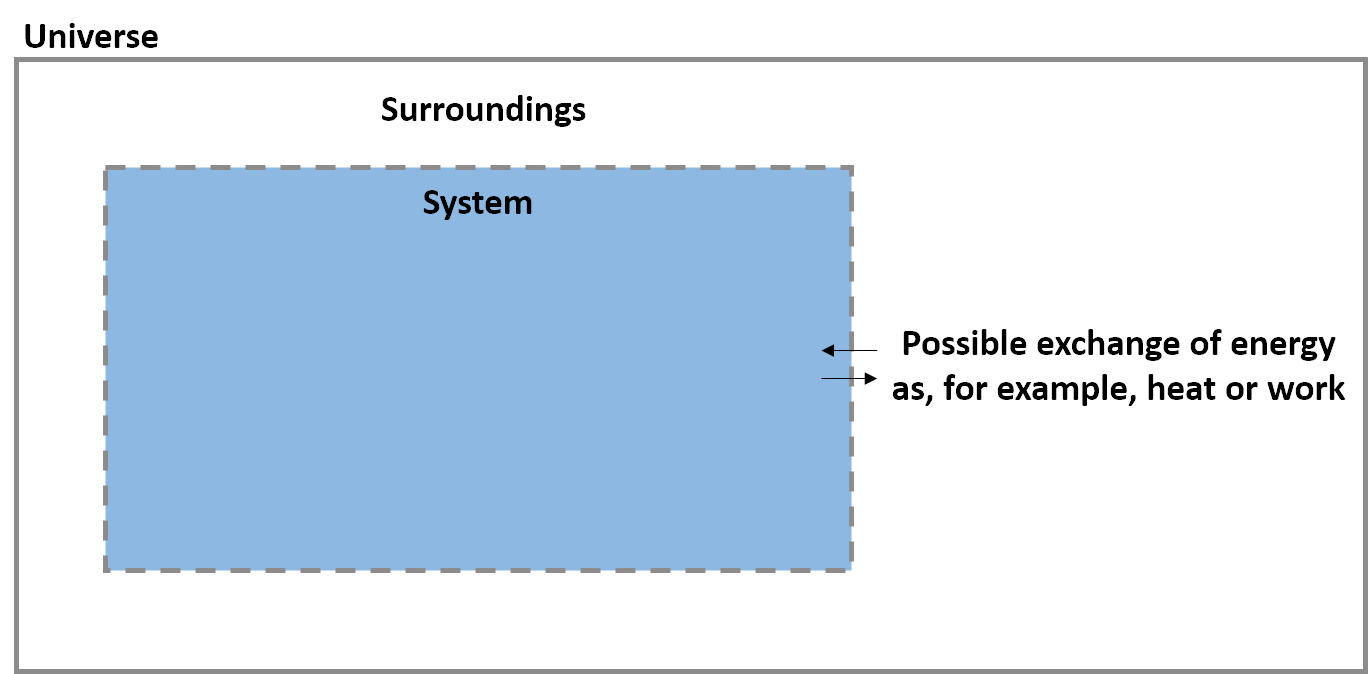
State variables describe equilibrium (static) conditions where nothing is changing. This is not particularly useful for chemists, where processes and changes (reactions) are more relevant than equilibrium states. Thermodynamics can also be used to describe changes in state.
The easiest way to change the state of a system is to move energy across the boundary between the system and surroundings. This transfer of energy is called heat (q) when it is the result of a difference in temperature and work (w) when it is the result of a force applied over a distance. Heat and work are examples of energy fluxes.
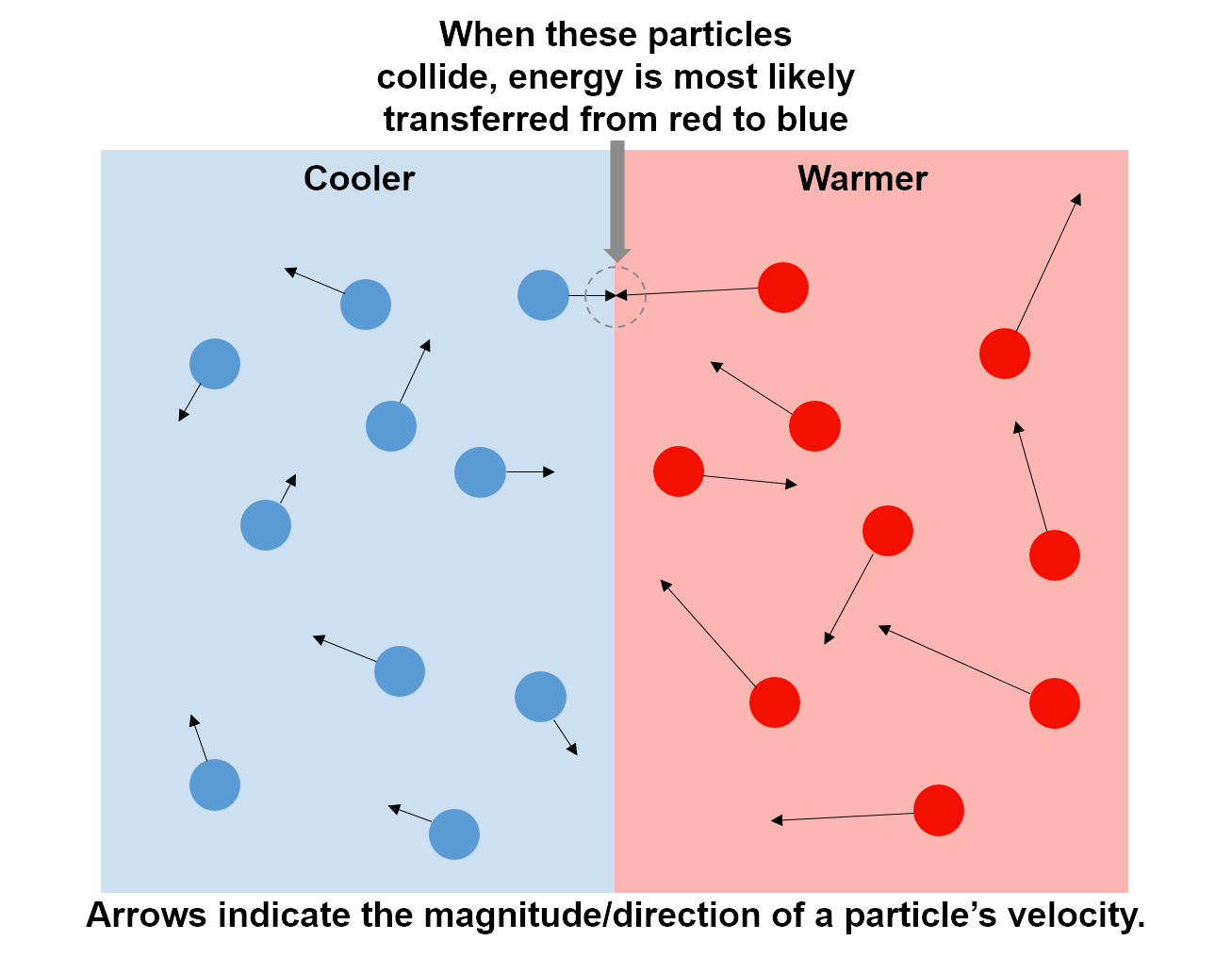
Heat always flows from a warmer object to a cooler object. For example, if you wrap your hands around a freshly brewed mug of coffee, you can feel heat transfer from the mug (warmer) to your hands (cooler). To conceptually understand how energy is transferred as heat, let's consider this example in more detail. Temperature is related to kinetic energy, such that the particles (molecules) in a system with higher temperature have a higher average kinetic energy than the particles in a system with lower temperature. That is, on average the particles in the mug (warmer) have more kinetic energy than the particles in your hand (cooler). Thus, when the molecules on the outside of the mug (higher average kinetic energy) collide with the particles on your skin (lower average kinetic energy), kinetic energy will (on average) transfer from the mug to your hands. If you hold the mug for long enough, your hands will warm up and the mug will cool down until both are the same temperature. The same is true for any two systems with different temperatures: energy transfers from the warmer to the cooler object as heat until the two temperatures are the same.
In Chemistry 123, we will focus on work that results from a system that undergoes an expansion or compression. Such processes result in changes in pressure (P) and/or volume (V), so the resulting work is called PV-work. Other types of work (e.g. electrical work) are possible, but will not be discussed in this course. Anytime you see a reference to work in this text, you can assume that it is PV-work unless otherwise specified. When the volume of a system changes (ΔV) during a process while the external pressure (i.e. the pressure outside the system), Pext, is constant, the work is calculated as
\(w=-P_{ext} \Delta V\)The external pressure is sometimes also referred to as the opposing pressure, Popp. Work is usually reported in units of Joules (J), while pressure is in units of atmospheres (atm) or bars (bar) and volume is in units of litres (L), so the equalities \(101.33 \ J = 1 \ L \ atm\) and \(100 \ J = 1 \ L \ bar\) can be helpful if you need to convert units. The negative sign is necessary to honour the sign conventions for heat and work. Whenever heat or work result in an addition of energy to the system, they have positive values. When heat or work result in a loss of energy from the system, they have negative values. Thus, when heat flows into the system q>0 and when heat flows out of the system q<0. When a system is compressed w>0 and when a system is expanded w<0. Since the final volume is larger than the initial volume in an expansion, ΔV>0 and thus the negative sign in the formula above results in a value of work that is negative (note that pressure values are always positive).
Try it: Indicate the sign of heat and/or work for each process below:
All processes in thermodynamics that we will discuss in CHEM 123 have the same form. The system begins in one equilibrium state, described by one set of state variables (called the initial state). Then, the system undergoes a process where heat or work is exchanged with the surroundings (note that the system may or may not be in equilibrium during this process). Finally, the system reaches another equilibrium state that is described by another set of state variables (called the final state).
\(Initial \ state \overset{Process}{\rightarrow}Final \ state\)When a system undergoes a process, the change in state variables depends only on the initial state and final state of the system, not on the path (i.e. how the system changes from the initial state to the final state). Thus, the change in any state variable over the course of a process is simply the difference between its final and initial values. If we represent a generic state variable with "X", then the change in this state variable, ΔX (read: "delta X"), is given by the difference between the final value of X, Xf, and the initial value of X, Xi:
\(\Delta X=X_{final}-X_{initial}=X_{f}-X_{i}\)Sometimes the initial and final states are referred to by numbers. For example, if a system undergoes a change of state from state 1 to state 2, then ΔX may be written as
\(\Delta X=X_{2}-X_{1}\)Energy fluxes like heat and work, described above, do vary depending on the path. Variables like heat and work that depend on the path between two states are called path variables or process variables.
Let's use temperature (a state variable) as an example to understand how two processes that start and end in the same state will have the same temperature change, even though their paths are different.
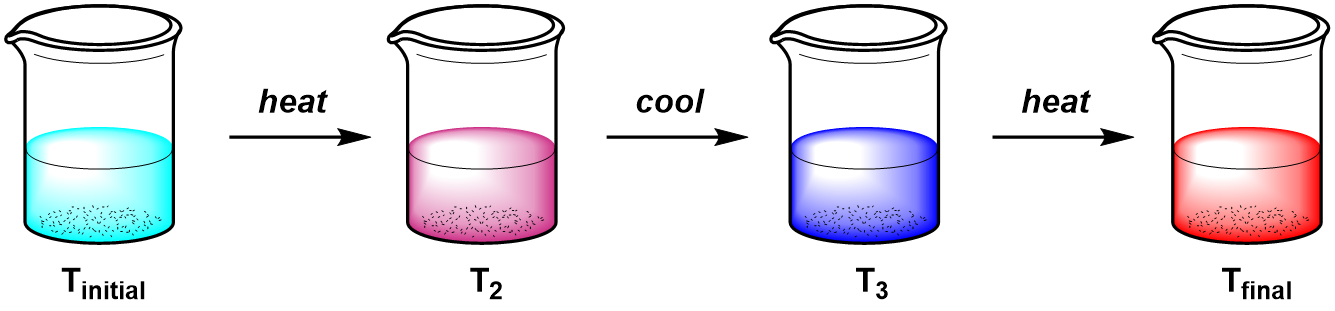
Process 1:
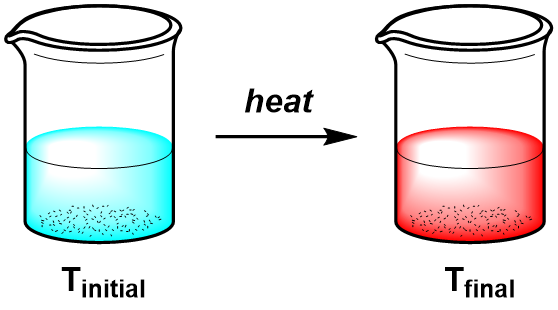
Process 2:
In process 1, a system is heated (to T2), then cooled (to T3), then heated again to Tfinal. The overall change in temperature only depends on the initial temperature, Tinitial, and the final temperature, Tfinal and is calculated as
\(\Delta T=T_{final}-T_{initial}\)In process 2, the same system is heated directly to Tfinal. The ΔT for both processes is the same because the overall change in temperature (ΔT) does not depend on how many different temperatures (or in what order) the system passes through.
Heat is a process variable, so in the example above the total amount of heat, q, transferred to the system (during heating) and from the system (during cooling) depends on the path. Temperature is a state variable, so the total change in temperature, ΔT, is independent of the path taken.
Interactive: