Molecules can rotate freely about σ-bonds, as we studied in the previous section on conformations. A double bond, however, is not able to freely rotate since doing so would break its π-bond. In this section we will study the distinct isomers of double bonds that can result from this lack of rotation, including how to distinguish the isomers using cis/trans and E/Z nomenclature. Double bond isomers play an important role in vision, the relative benefit/harm of unsaturated fats, and the elasticity of polymers.
The conversion between two isomers of retinal plays a key role in the vision cycle of humans and other mammals. Our bodies convert Vitamin A into 11-cis-retinal molecules, which form the chromophores (along with opsins) in our eyes. When photons enter our eyes, they excite the electrons in the π-bond of 11-cis-retinal highlighted in red below, temporarily breaking the double bond and leaving a single bond that is free to rotate. The molecule rotates about this single bond, then the double bond reforms to make all-trans-retinal. The isomerism of 11-cis-retinal to all-trans-retinal significantly changes the shape of the retinal molecule and drives the continuation of the vision cycle.

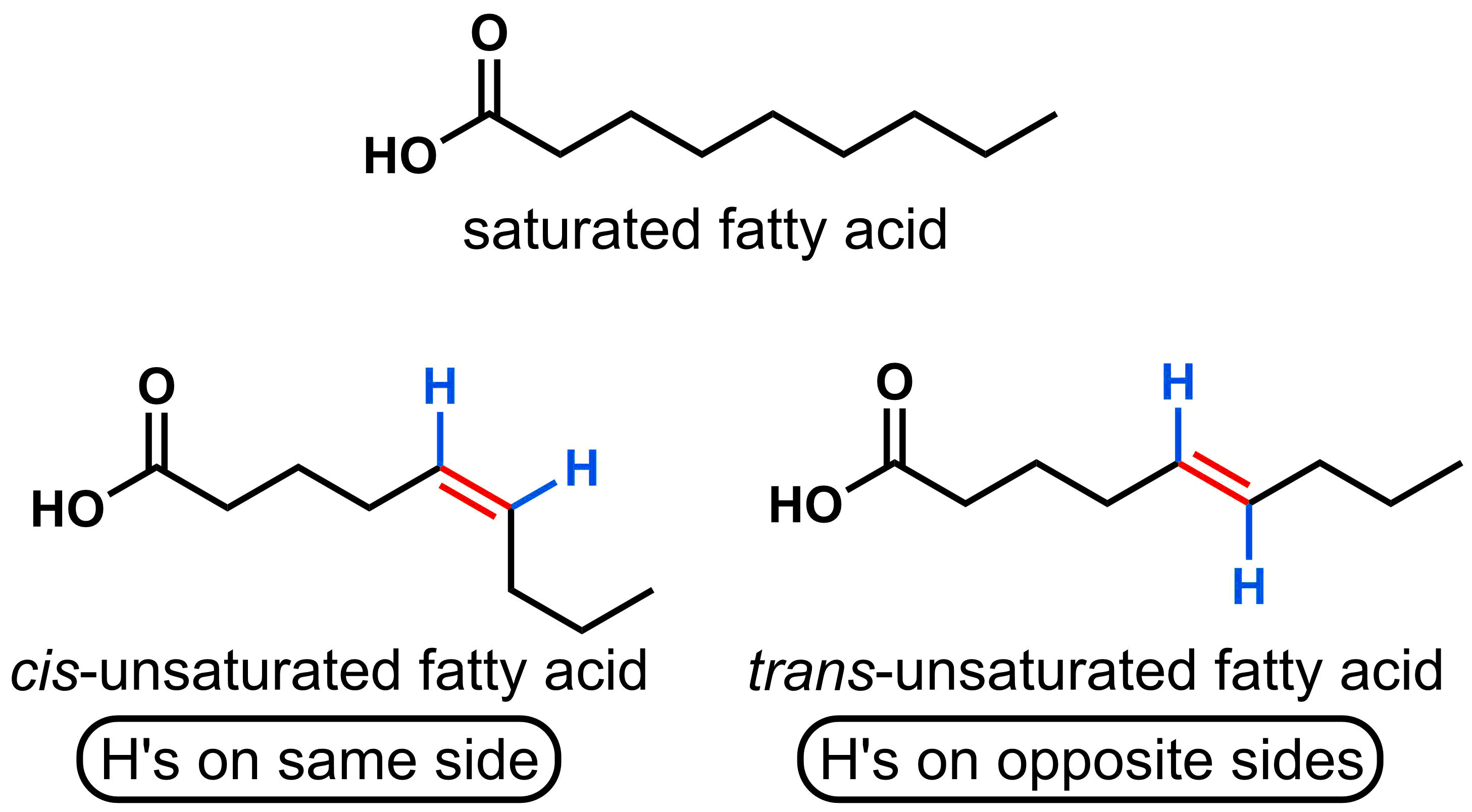
Fatty acids consist of a carboxylic acids with long hydrocarbon chains. If the hydrocarbon chain contains single bonds only, then the fat is classified as saturated, and if it contains any double bonds then we classify it as unsaturated. If the hydrogens attached to the double bonded carbons are on the same side of the molecule, then it is a cis-unsaturated fatty acid, and if they are on opposite sides of the molecule then it is a trans-unsaturated fatty acid. Naturally occurring unsaturated fats are made of mostly cis-unsaturated fatty acids, for example vegetable oil, and they tend to be liquids at room temperature. Saturated fats, by contrast, are usually solids at room temperature. Trans-unsaturated fats are usually a byproduct when converting cis-unsaturated fats into saturated fats, which manufacturers do to prolong shelf life (oils go rancid most quickly than solid fats) or make fats with 'desireable' melting points such as margarine. It is trans-unsaturated fat that has been linked to an increased risk of coronary heart disease.
The two isomers (cis and trans) of butadiene can both be polymerized, but they produce polymers with very different properties. Cis-polybutadiene is a bouncy elastomer, often used in the rubbery core of golf balls. In contrast, trans-polybutadiene is very hard with high tensile strength and would be better suited for use in the outside of golf balls.

|
 |