- Molecules can rotate around σ-bonds but not π-bonds.
- Conformers are identical species (i.e. not isomers) that differ only through σ-bond rotation.
- Stereoisomers have the same molecular formula and the same connectivity, but differ in the arrangement of their atoms in space.
To understand alkenes and their isomers, it is first important to have a thorough understanding of their structure and bonding. According to Valence Bond theory, each carbon of the alkene is sp2-hybridized and is trigonal planar. To form a π-bond, the two remaining p-orbitals on each of the sp2-carbons must be coplanar. A consequence of two adjacent trigonal planar atoms that must have coplanar p-orbitals is both atoms in the alkene and the four atoms connected to the alkene must all lie in the same plane.
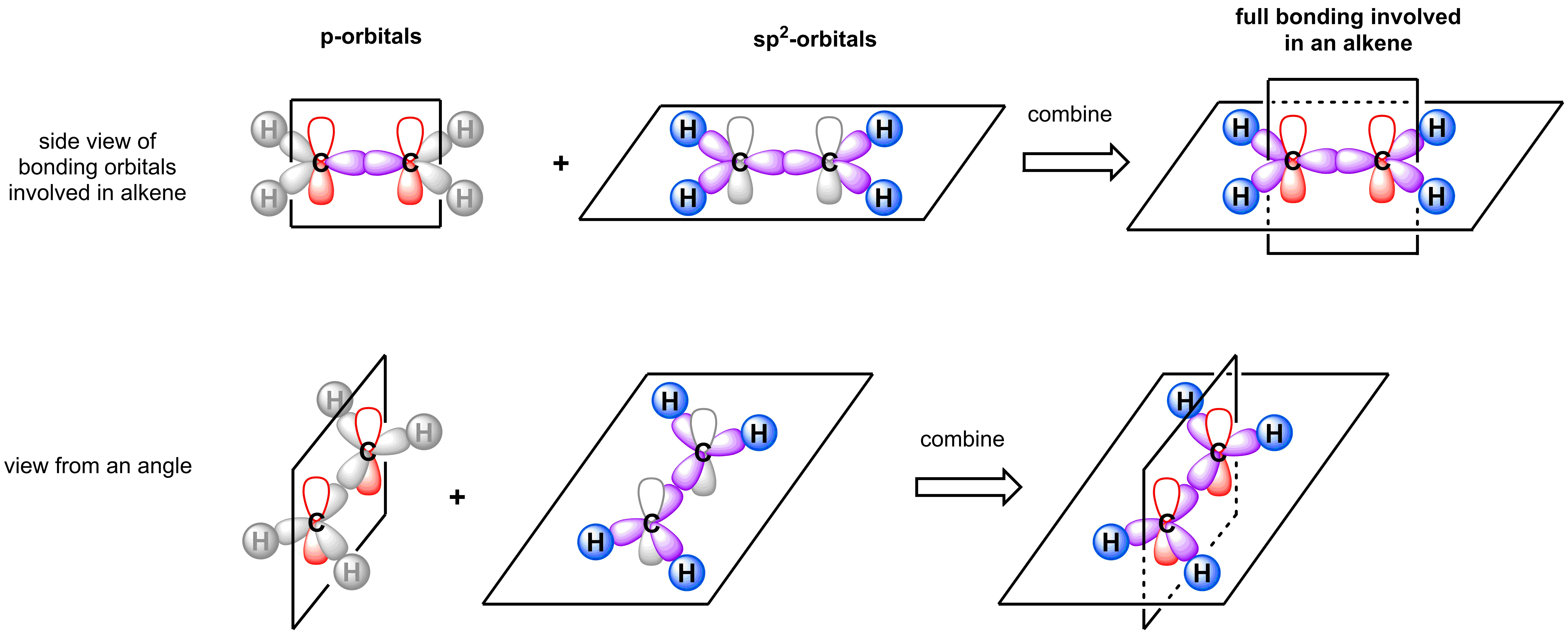
While rotation about any of the single bonds connected to the alkene is possible, rotation about the alkene itself poses a problem. Any attempt to rotate about the alkene results in a change in the relative position of the p-orbitals from coplanar to orthogonal. This eliminates any overlap, and results in the breaking of the π-bond. At moderate temperatures, this rotation will not occur and, therefore, the alkene is conformationally locked (see video below).
An important consequence of restricted rotation can be seen with 2-butene. There are two ways to draw 2-butene: one in which the methyl groups are on the same side of the alkene and one in which the methyl groups are on the opposite sides of the alkene. Because there is no rotation about the π-bond, these two molecules do not interconvert.
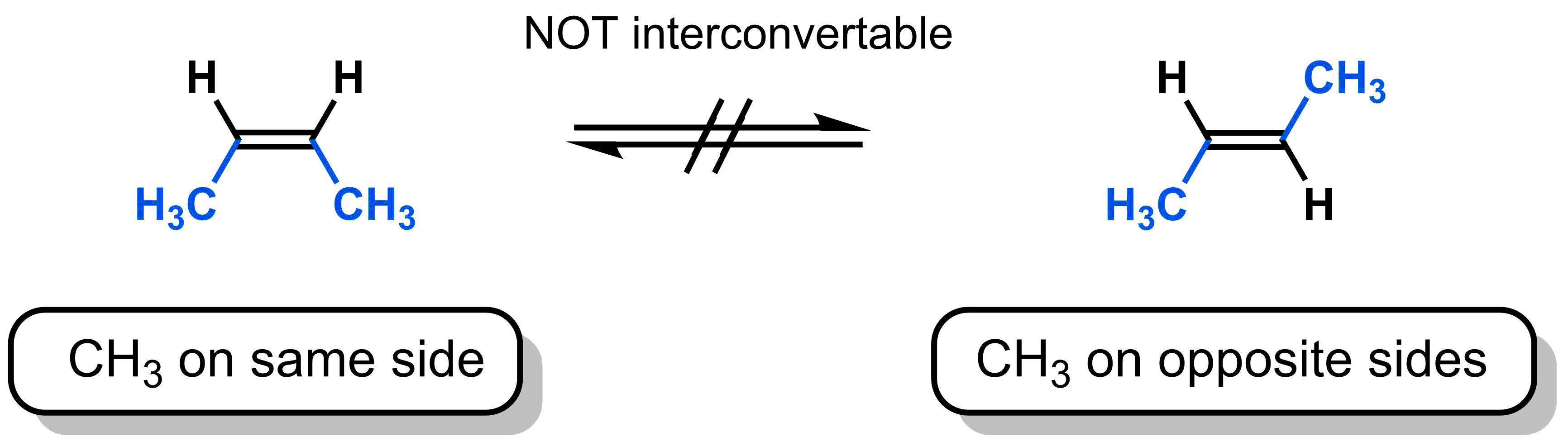
These two 2-butene forms have the same molecular formula and the same connectivity, but cannot interconvert. These two molecules represent a new type of isomer called a stereoisomer, with the following definition:
Stereoisomers have the same molecular formula and the same connectivity, but differ in the arrangement of their atoms in space.
The two molecules are different, but have the same name (2-butene). Therefore, additional nomenclature (presented in Sections 1.2-1.4) is needed to differentiate them.
In summary, two molecules are the same if you can:
1. Rotate one of the molecules about its σ-bonds to make it look like the other (i.e. they are conformers) and/or
2. Rotate one of the molecules to make it look like the other.
If the two molecules have the same connectivity, but cannot be rotated to make the two look the same, then they are stereoisomers, as shown in the examples below.


Interactive: