- Fischer projections can efficiently depict molecules with many stereocentres.
- Vertical lines indicate groups going into the page (i.e. dashes) and horizontal lines represent groups coming out of the page (i.e. wedges).
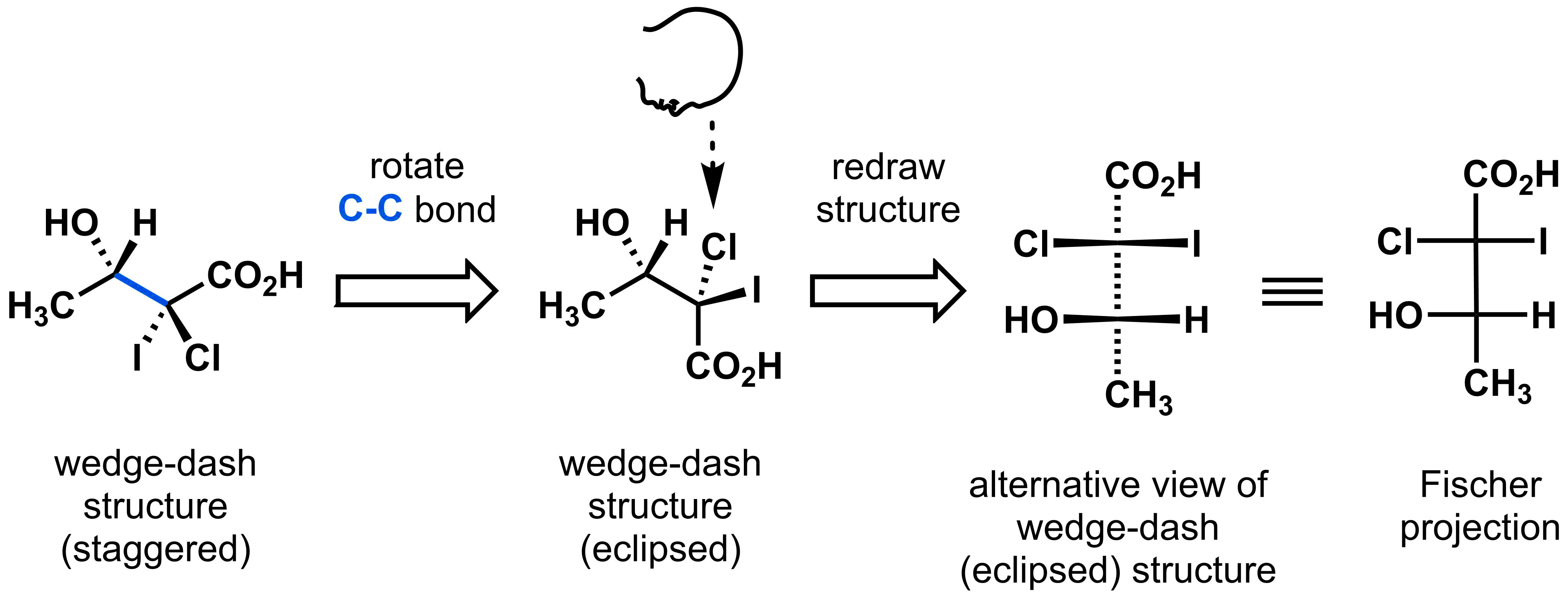
For most stereochemical analysis, wedge-dash diagrams and Newman projections will be the best ways to represent chemical structure. However, for a few, very specific applications, a Fischer projections can be very efficient. Fischer projections are a way of representing a molecule with a set of vertical and horizontal lines. In this representation, the vertical line indicates the bond going towards the back of the page (dashed bonds) while the horizontal line depicts a bond that is coming out of the page (wedged bonds).

To convert a wedge-dash structure with multiple stereocentres into a Fischer projection, begin by rearranging the molecule to obtain a wedge-dash structure in eclipsed conformation. This will put the substituents on the same side (i.e. either at the top as shown or at the bottom) and will make it easier to convert it into a Fischer projection. Then redraw the eclipsed, wedge-dash structure so that the highest priority carbon is at the top and the substituents point out of the page. You will now have your Fischer projection obtained.
Interactive:
Interactive (Challenge questions):