- Newman Projections show the 3D orientation of the atoms in a molecule by looking at a particular bond head-on rather than side-on.
In a Newman projection, a carbon-carbon bond is viewed head-on, so that one carbon atom is directly behind another. For example, consider model 1 of ethane, below. The carbon and hydrogen atoms on the left are highlighted in red, and those on the right are highlighted in blue to help you keep track of them. Imagine standing on the left side of model 1 and looking directly down the carbon-carbon bond. From this perspective, the model would look like model 2, with the carbon highlighted in blue hidden behind the carbon highlighted in red. This is the perspective we use when drawing a Newman projection. In a Newman projection, the front (red) carbon is represented by a central dot. The back (blue) carbon is represented by a larger circle. The substituents off of the front carbon (shown in red) are drawn at 120o with bonds connecting all the way to the center. The substituents off of the back carbon (shown in blue) are also drawn at 120o, but the bonds stop at the edge of the larger circle.
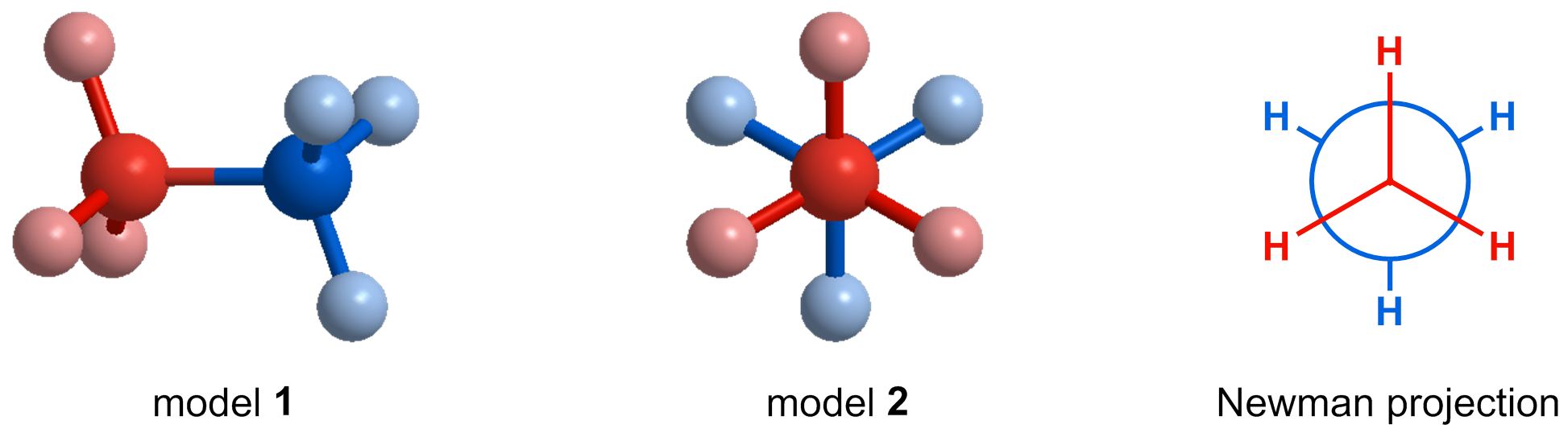
Try rotating the model below yourself to get a sense for the perspective we use when drawing a Newman projection.
Practice for this section not currently available