- Wedge-dash structures depict the 3D orientation of atoms, so can be used to represent conformers.
To study bond rotations, we need to use a molecular representation that can show the spatial arrangement of the atoms. Let’s consider the depictions of ethane that you have learned thus far in the course: condensed structural formula, Lewis dot structure, line-bond structure and wedge-dash structure. All of these representations equally show how the atoms in ethane are bonded together, but only the wedge-dash structure can reflect the spatial arrangement of atoms depicted in the model. Because molecular rotations affect the three dimensional structure of a molecule, we can use wedge-dash structures to keep track of the relative positions of atoms while studying rotations.
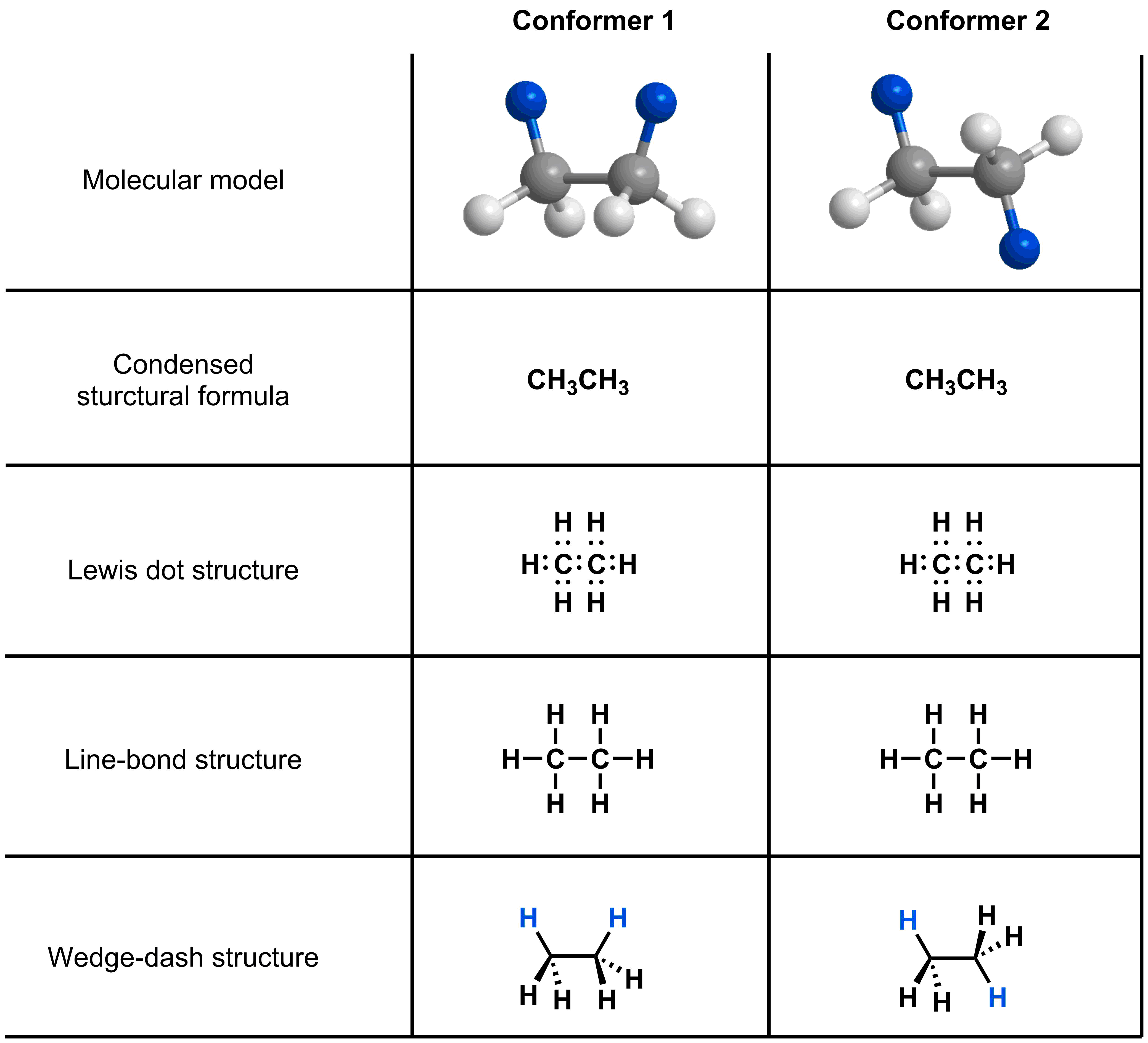
Click on the structure above to see the relationship between the molecular model and the wedge dash diagram.
Sometimes it is helpful to keep track of bond rotations by looking at a C-C bond from a 45° angle or head-on, rather than side-on. We can do this by using two new molecular representations: sawhorse projections (section 6.3) and Newman projections (section 6.4).