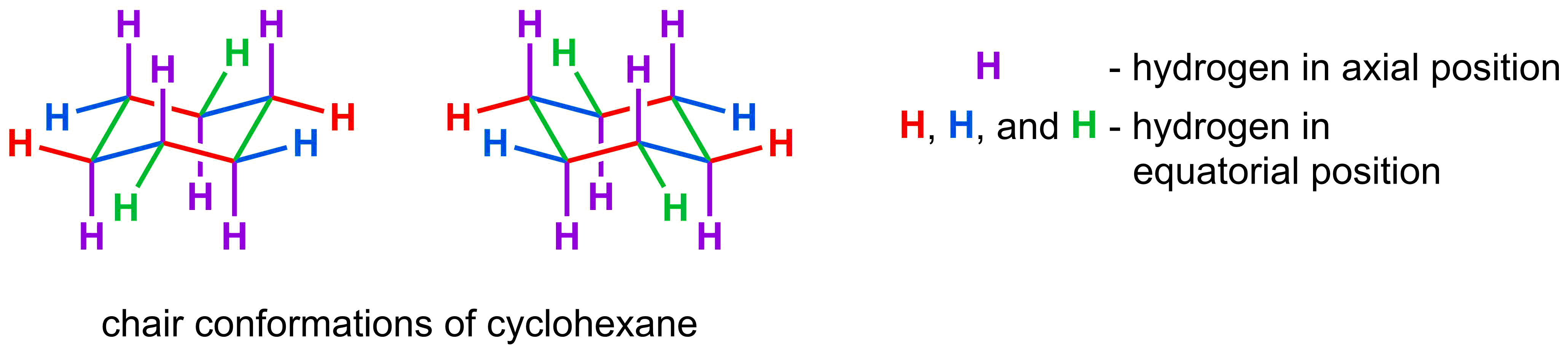
- The most stable conformation of a cyclohexane ring is a chair conformation
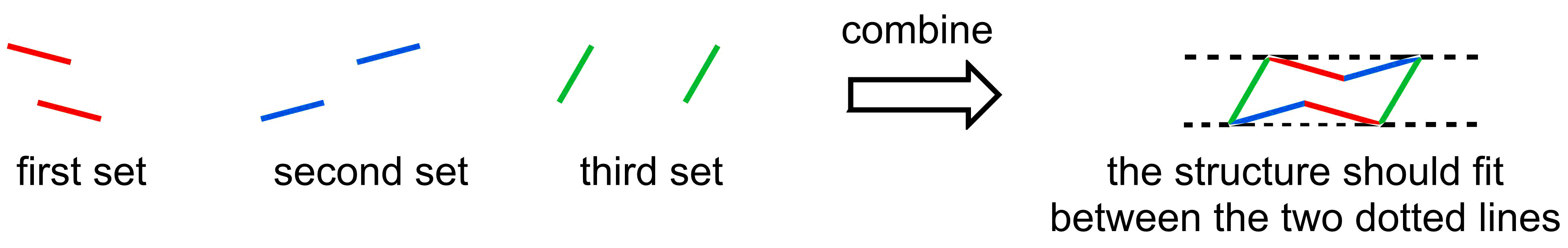
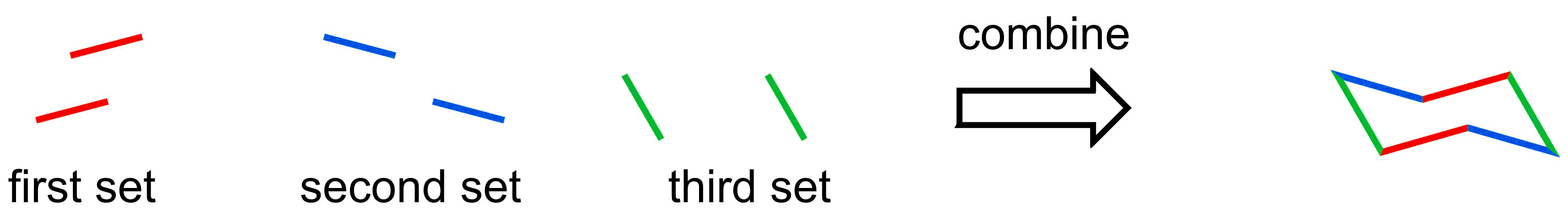
- The 2D representation of a chair conformation has 3 sets of parallel lines
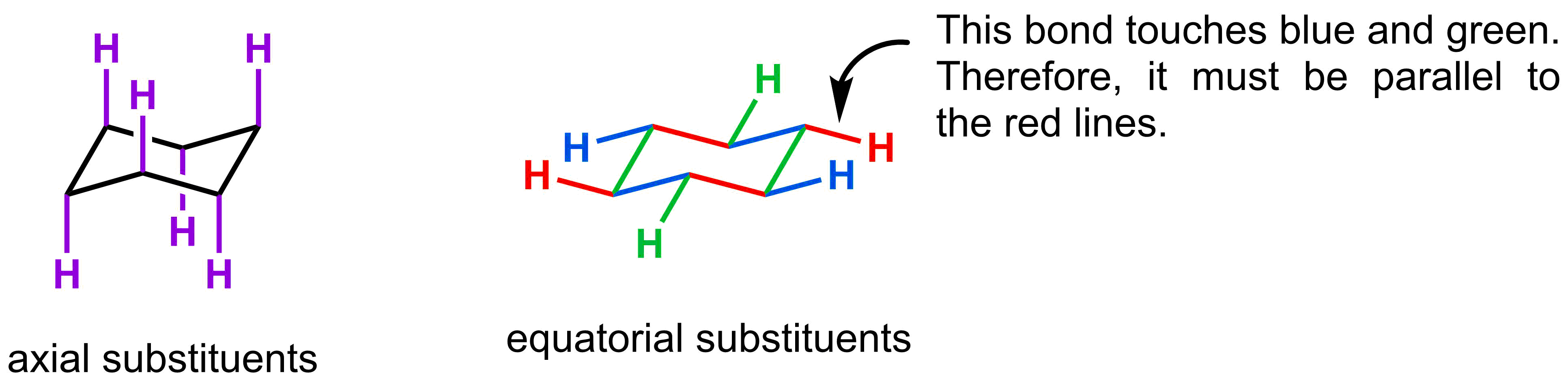
- Each carbon in a cyclohexane chair has an axial and an equatorial substituent
Before we analyze the rotations within cyclohexane derivatives, it is first important to learn how to draw the lowest energy conformation, the chair conformation, in 3D.
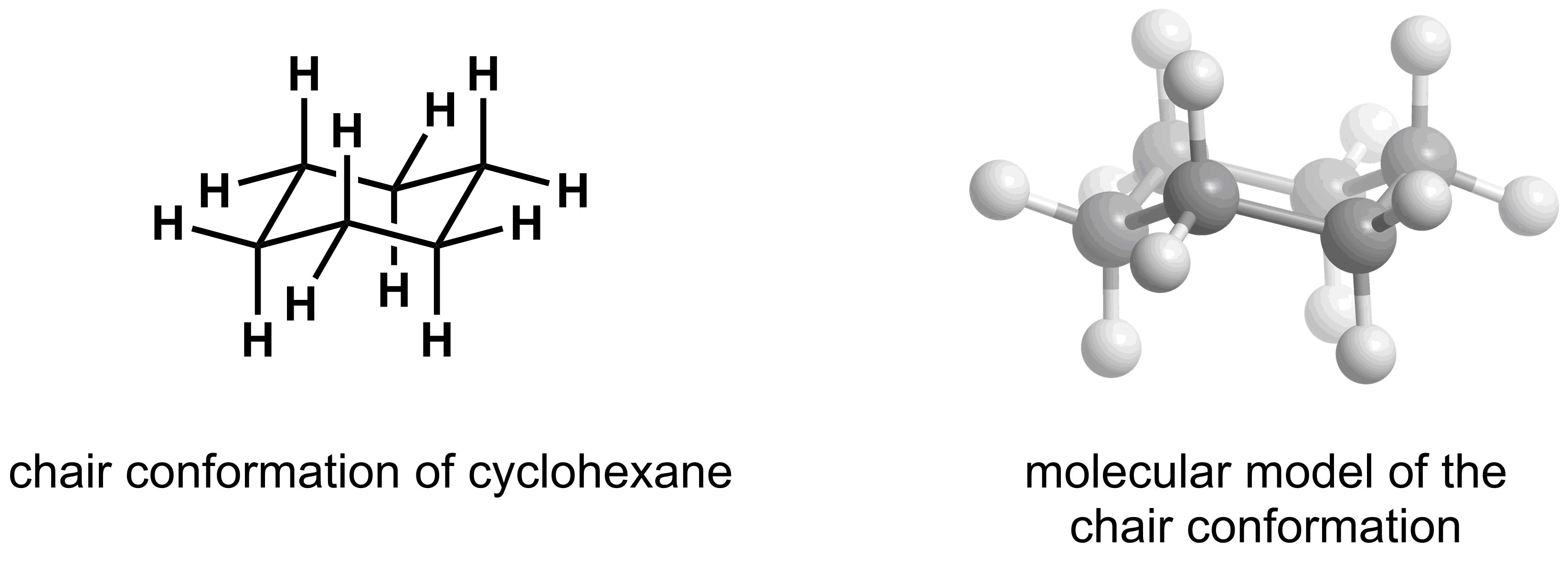
We are going to start by focusing on the carbon framework of the ring. The ring is depicted as three sets of parallel lines, where the two parallel lines are always opposite one another. Stylistically, the best chairs are drawn so that the chair can be drawn between the two dotted horizontal lines.
Alternatively, a chair structure can also be drawn where the first set of parallel are mirrored (i.e. point down from right to left rather than left to right).
Next, we are going to focus on the substituents on carbon. There are two different types of substituents: axial and equatorial. Axial substituents are the easiest to draw as they simply drawn alternating straight up and straight down around the ring. The equatorial substituents are in the meridional position and also alternate either up or down. They are drawn so that they are parallel to two lines in the ring. To determine which two lines they are parallel to, look to see where the substituent is attached. That substituent is parallel to the color of the lines it is not touching.
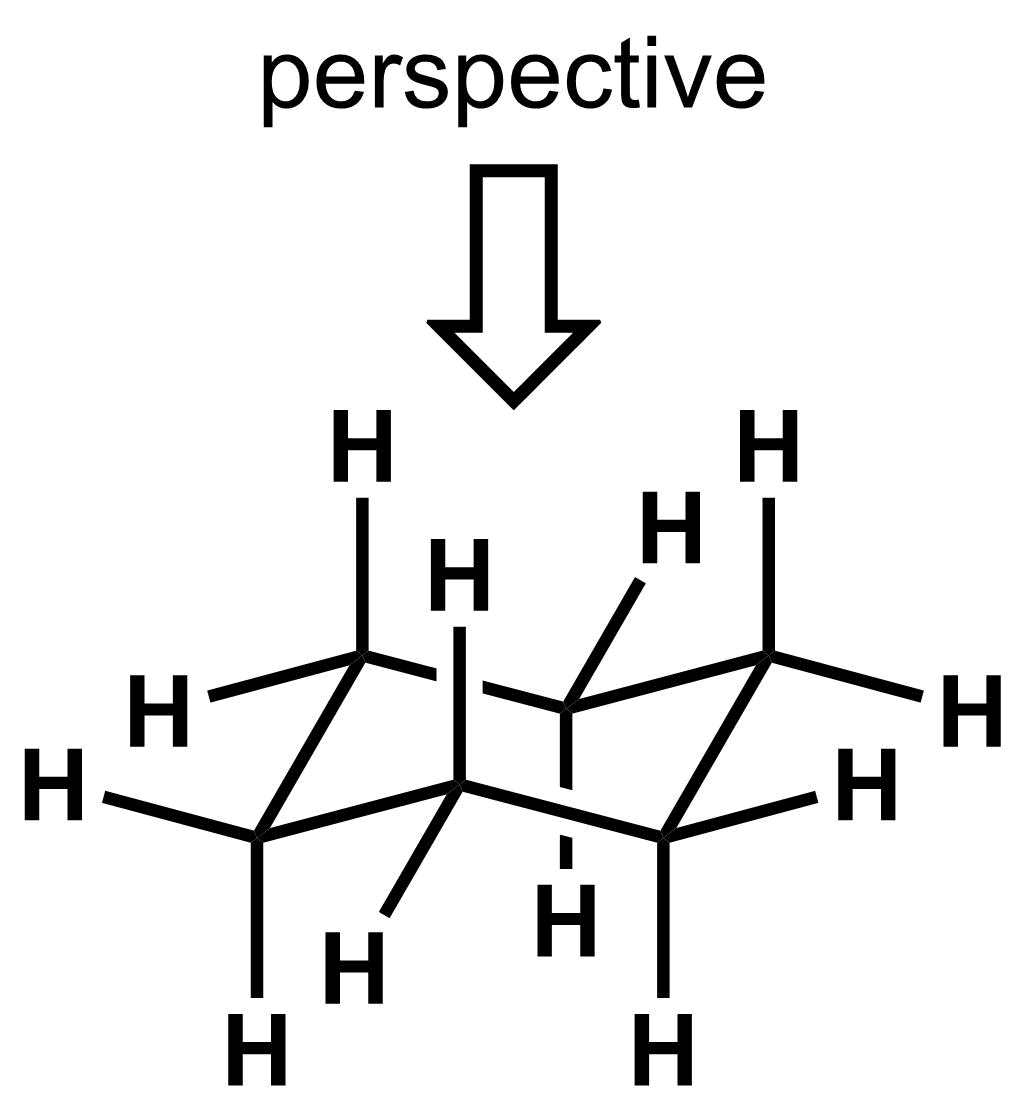
Taken all together, the chair conformation for cyclohexane can be drawn in either 3D representation as shown below.
During our analysis of chair cyclohexane, it is important to be able to determine the relative positions of all the substituents. We are going to define that when a cyclohexane is drawn in the chair conformation, everything closer to the top of the page is “up” and everything closer to the bottom of the page is “down”, which implies that up is defined from a top-down view. Please keep in mind that “up” and “down” are purely used to determine the relative orientation of the substituents.
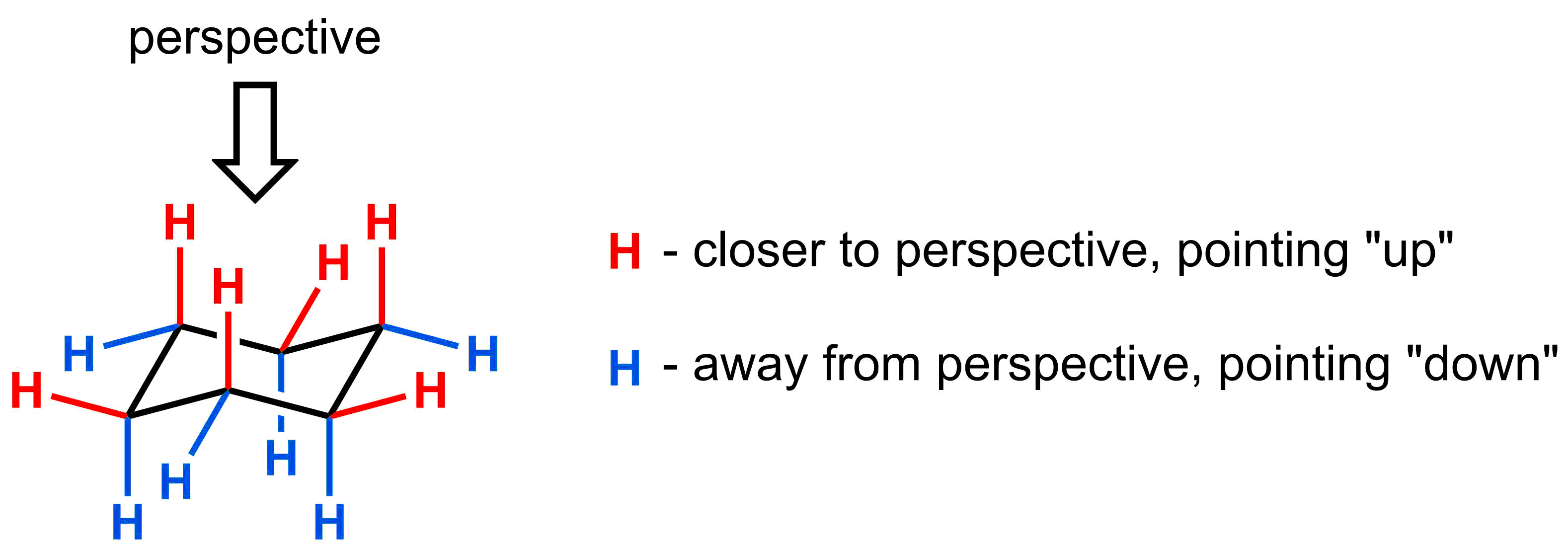
To determine what is “up” and what is “down”, just look at the two substituents on any carbon. If the substituent is closer to the indicated perspective, the substituent is “up”. If the substituent is farther from the indicated perspective, the substituent is “down”.
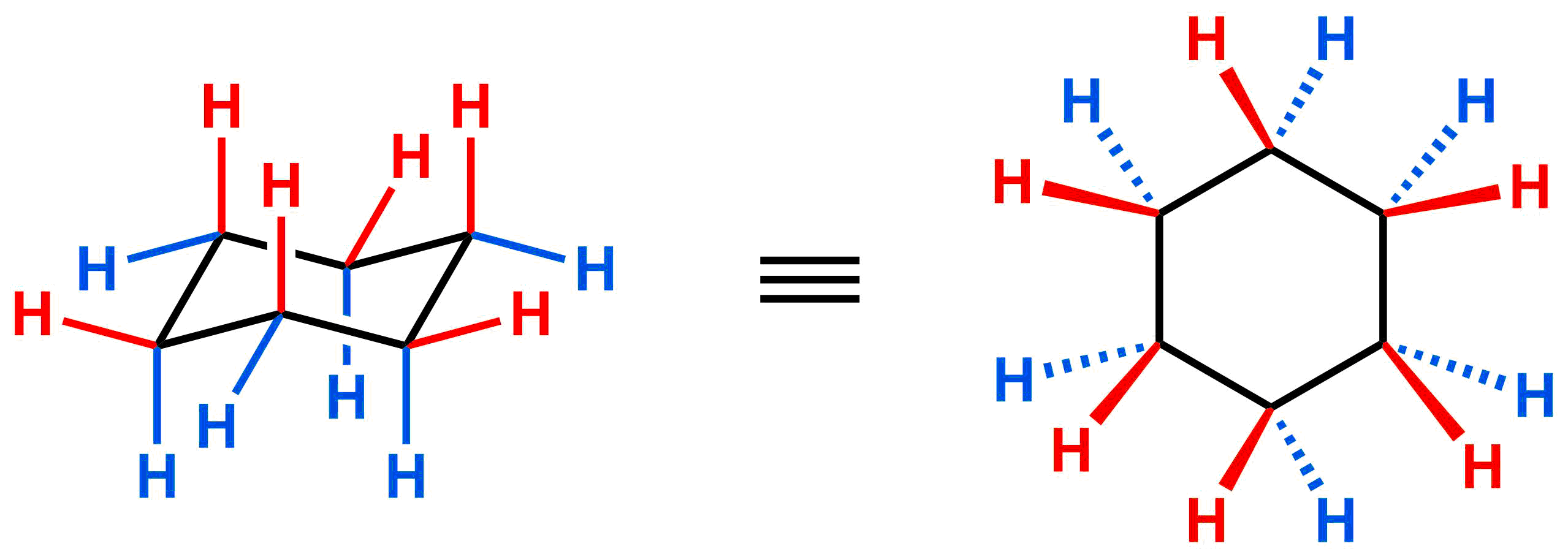
This is useful because it allows us to directly correlate the chair conformation to a 2D depiction of cyclohexane. Everything that is “up” is on the same side of the 2D depiction (in red) and everything that is “down” is on the same side.
Rotate the cyclohexane model below to explore how these 2D representations correspond to the 3D structure for yourself.
Interactive: