- We can draw a double Newman projection of a cyclohexane by looking down two parallel bonds simultaneously.
When we examine bond rotations within 6-membered rings, it will be important to be able to visualize and relate the molecule back to all of your representations of molecular structure. Lets start by visualizing chair cyclohexanes as Newman projections. Previously, we only looked down along one carbon-carbon bond to draw our Newman projection. For chair cyclohexanes, a far more useful view is to look down two bonds as a double Newman projection. To draw these diagrams, first find the two sets of parallel lines that will serve as the foundation of the double Newman, an example of which is provided below. Everything attached to the front carbons are indicated in red, everything attached to the back carbons are indicated in blue and the bolded bonds are used to indicate the viewing axis.
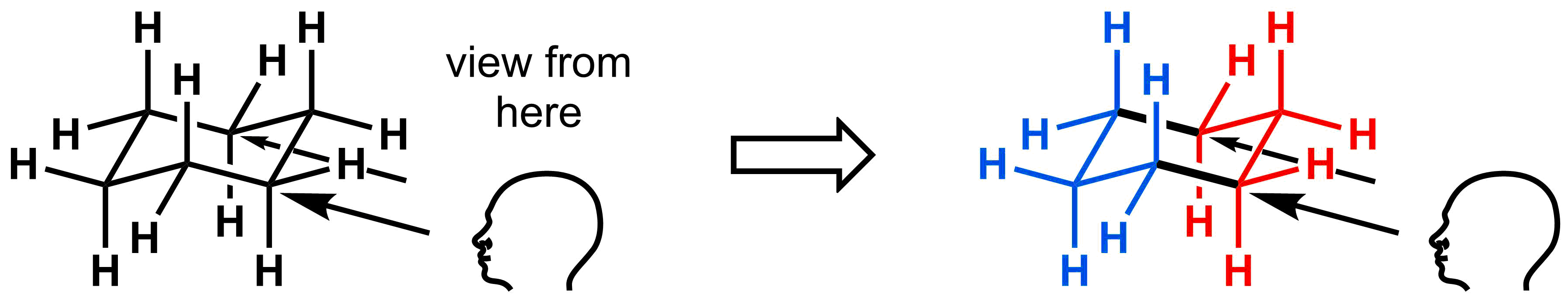
To draw the double Newman, start with two standard Newman projections side-by-side. Then, draw the axial hydrogens at both the front and back carbons. In the diagram below, the axial hydrogens are pointing down from the carbon atoms in the front (indicated in red) and pointing up for the carbon atoms at the back (indicated in blue). The rest of the Newman projection can be drawn by drawing the other bonds at 120° angles from each of the axial hydrogens. In these diagrams, it is not necessary to draw the hydrogens at the front (C1) and back (C4) corners of the diagram.
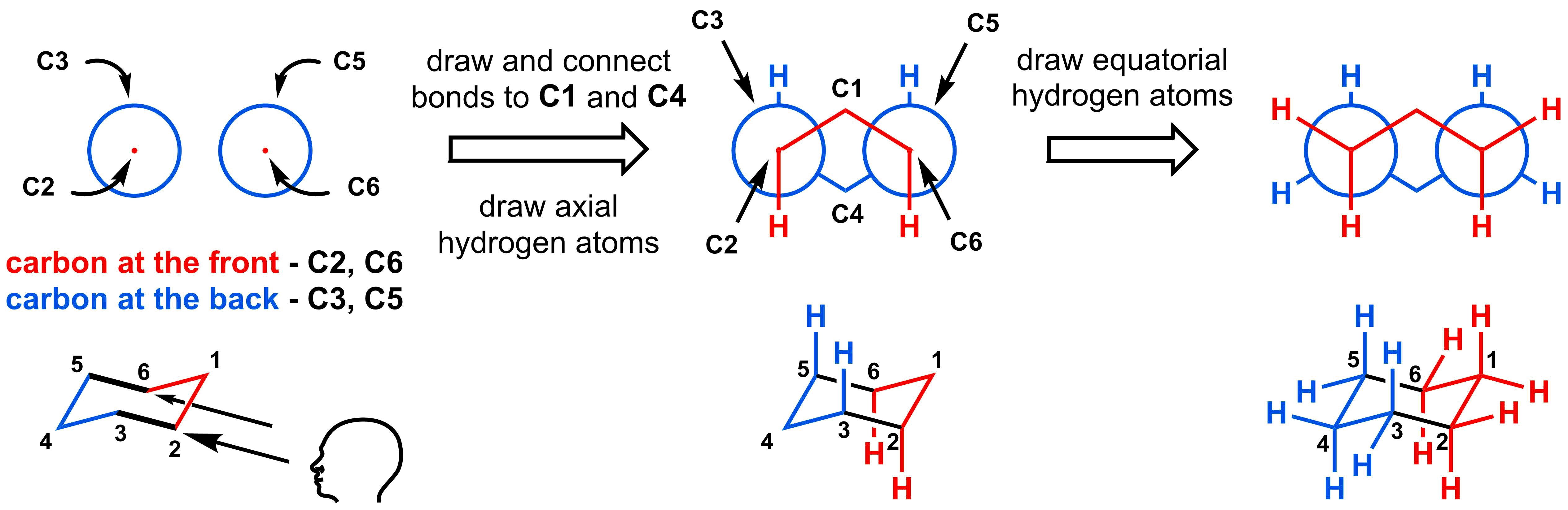
You may find the molecular model below helpful to see how the 3D structure corresponds to the 2D representation in the double Newman projection:
Interactive: