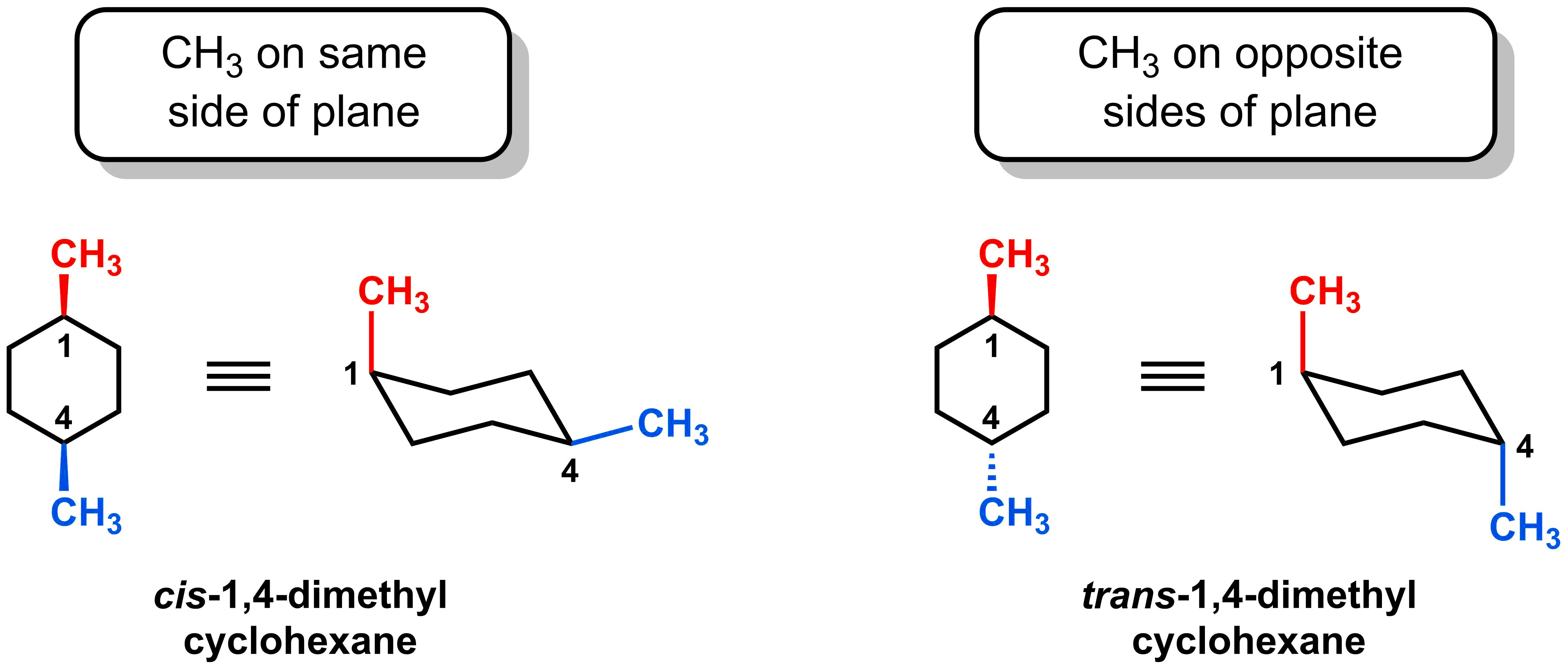
- Two substituents on the same side of a chair conformation have a cis-relationship
- Two substituents on opposite sides of a chair conformation have a trans-relationship
- It is less stable to have large groups close together due to steric interactions (e.g. 1,3-diaxial interactions)
Disubstituted cyclohexanes provide an additional level of difficulty because, not only do you need to consider 1,3-diaxial interactions between substituents and hydrogens, but now you also need to consider the interaction between two substituents. Before we analyze the energetic ramifications of disubstitution, we need to first introduce some new nomenclature. Lets examine the following two molecules, both of which are 1,4-dimethyl cyclohexane derivatives. As you can see from the two chair conformations, these are clearly not the same molecules, so additional information is required.
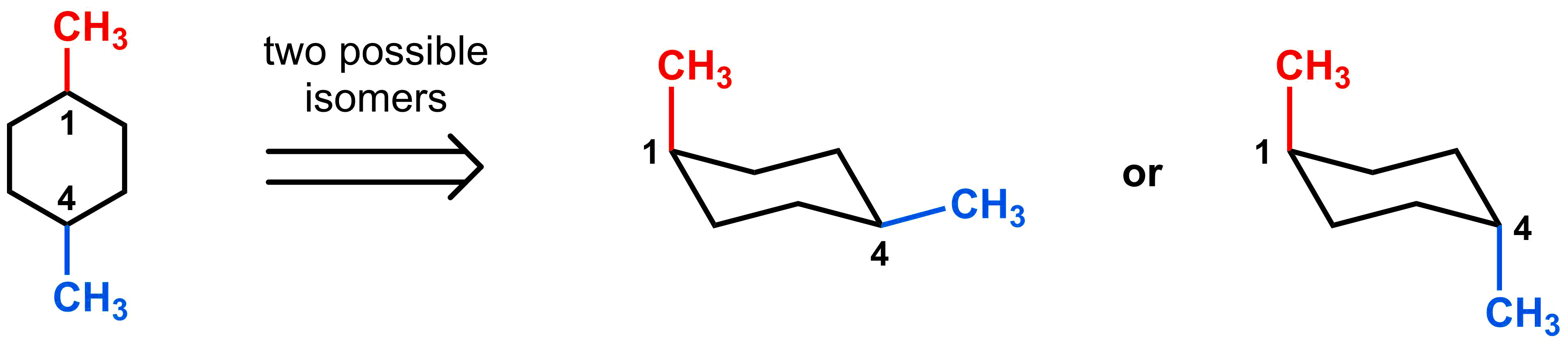
For these derivatives, we can introduce new nomenclature for the relative positioning of the two methyl groups. If the two methyl groups are on the same face of the cyclohexane (i.e. both up or both down), they are “cis”. If the two methyl groups are on opposite faces of the cyclohexane (i.e. one up and one down), they are “trans”. Thus, one can name the cyclohexane on the left cis-1,4-dimethyl cyclohexane and the molecule on the right trans-1,4-dimethylcyclohexane. Alternatively, you can refer to the two methyl groups on the left to be cis to each other and trans to each other on the right.
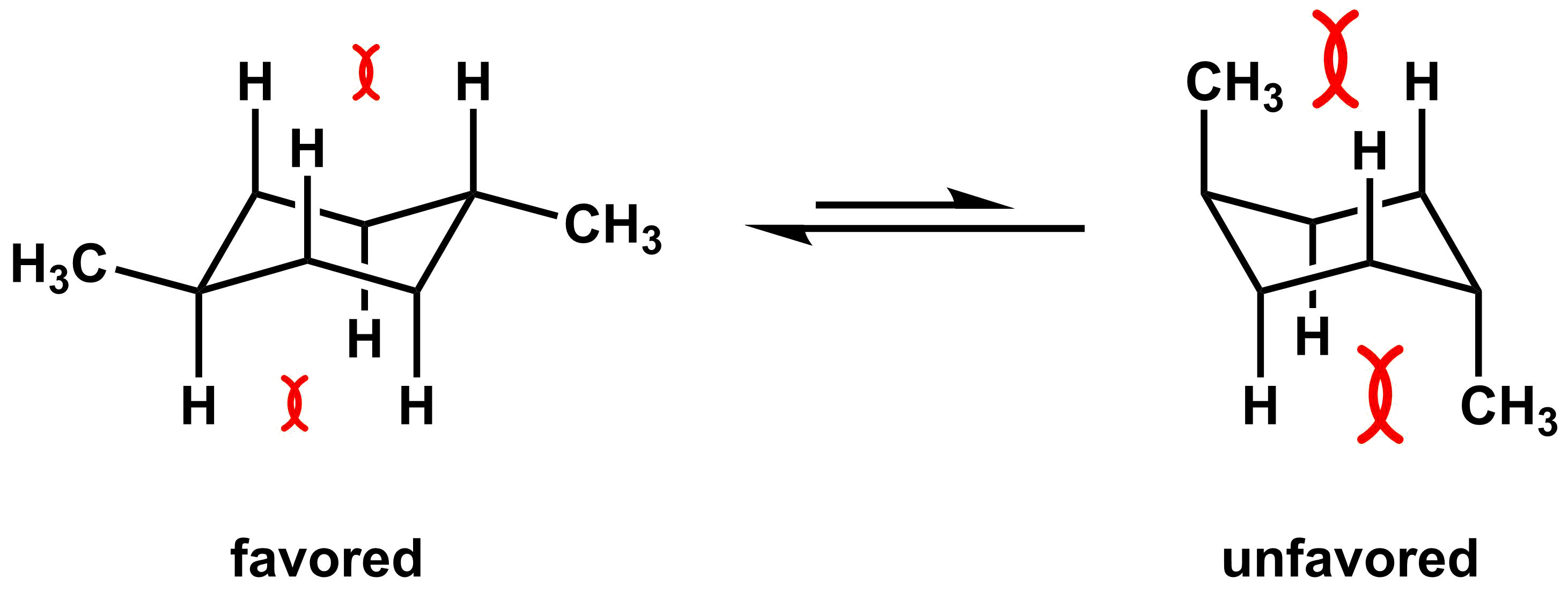
To compare energies of disubstituted cyclohexane derivatives, compare the relative energies of each substituent, and the conformation with the lowest steric interactions will be favored. Some derivatives, such as trans-1,4-dimethylcyclohexane are easy to determine which conformer is more stable. In the diequatorial conformation, there are no major 1,3-diaxial interactions, while the diaxial conformation has two methyl groups with significant 1,3-diaxial interactions. Thus, the conformer on the left is favored.
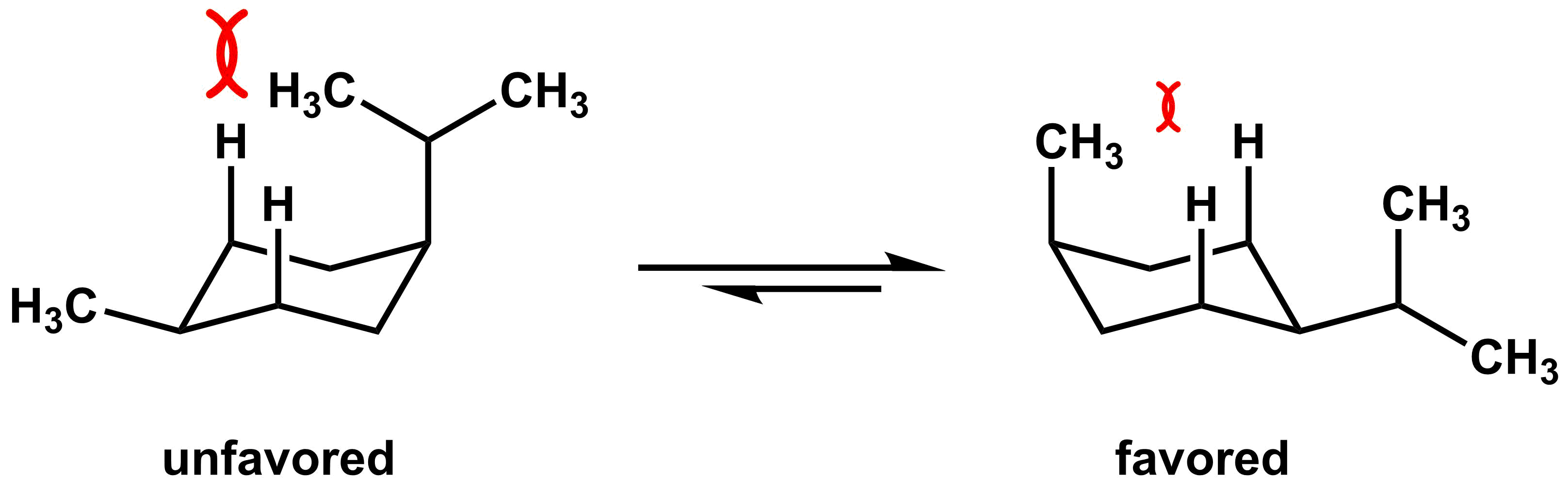
We can use the same type of analysis to analyze cis-1,4-disubstituted cyclohexane derivatives. For example, in the cyclohexane derivative below one of the chair conformations has the methyl group equatorial and the isopropyl group axial (on the left). In the other chair conformation, the methyl group is axial and the isopropyl group is equatorial. As we have previously seen, the isopropyl group leads to more significant 1,3-diaxial interactions, so the conformer on the left is higher in energy. Therefore, the conformer on the right is more stable and will be more prevalent in solution.
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
Interactive: