- Cyclohexane can adopt two different chair conformations.
- These two chair conformations rapidly interconvert at room temperature via a chair flip.
- When a cyclohexane chair flips from one chair conformer to another, all axial substituents become equatorial and vice versa.
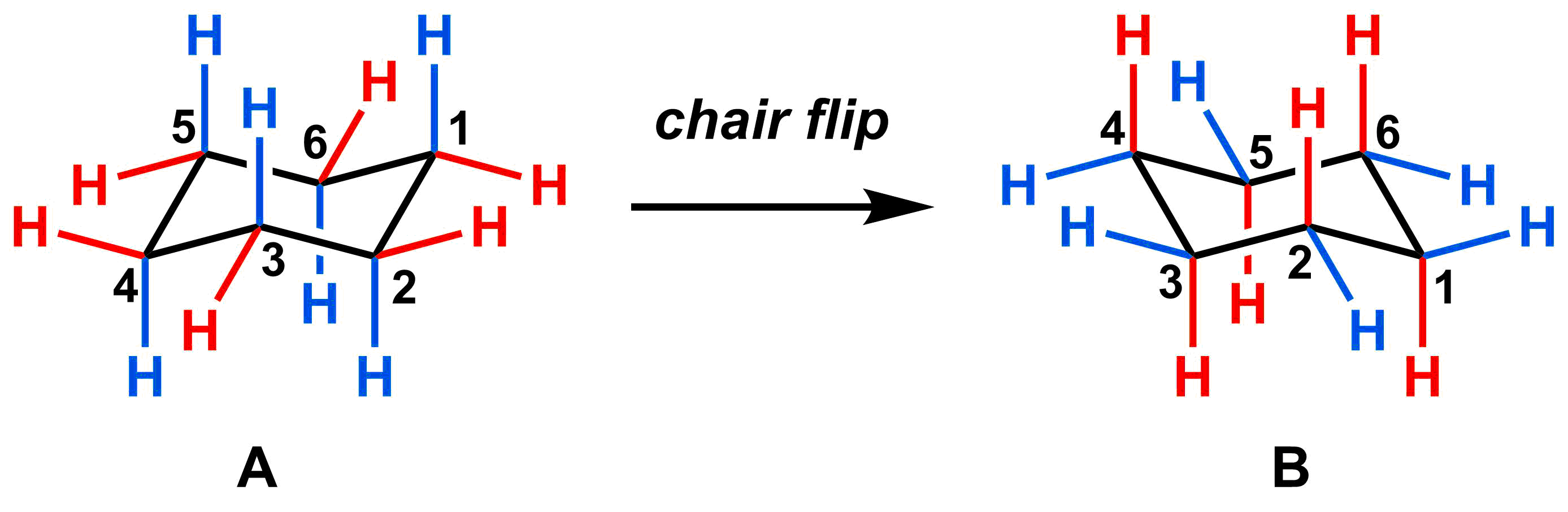
Now that we have several different representations for depicting and understanding cyclohexane, we can start our analysis of bond rotations. Because of the restricted rotation about each of the carbon-carbon bonds, full rotation is impossible. However, partial rotation is possible, which leads to different cyclohexane conformations. In the video below, we have labelled all of the substituents that start in the axial position in blue, and all of the substituents that start in the equatorial substituents in red.
As you can see, two different chair cyclohexane conformations are possible (A and B). The rotation from one chair conformation to the other is called a chair flip. As the chair flip occurs, the substituents that are initially axial (blue) become equatorial, and the substituents that are initially equatorial (red) become axial. While all axial substituents become equatorial and vice versa, whatever is in the “up” position for A remains in the “up” position in B. Similarly, the “down” position for A remains “down” position in B.
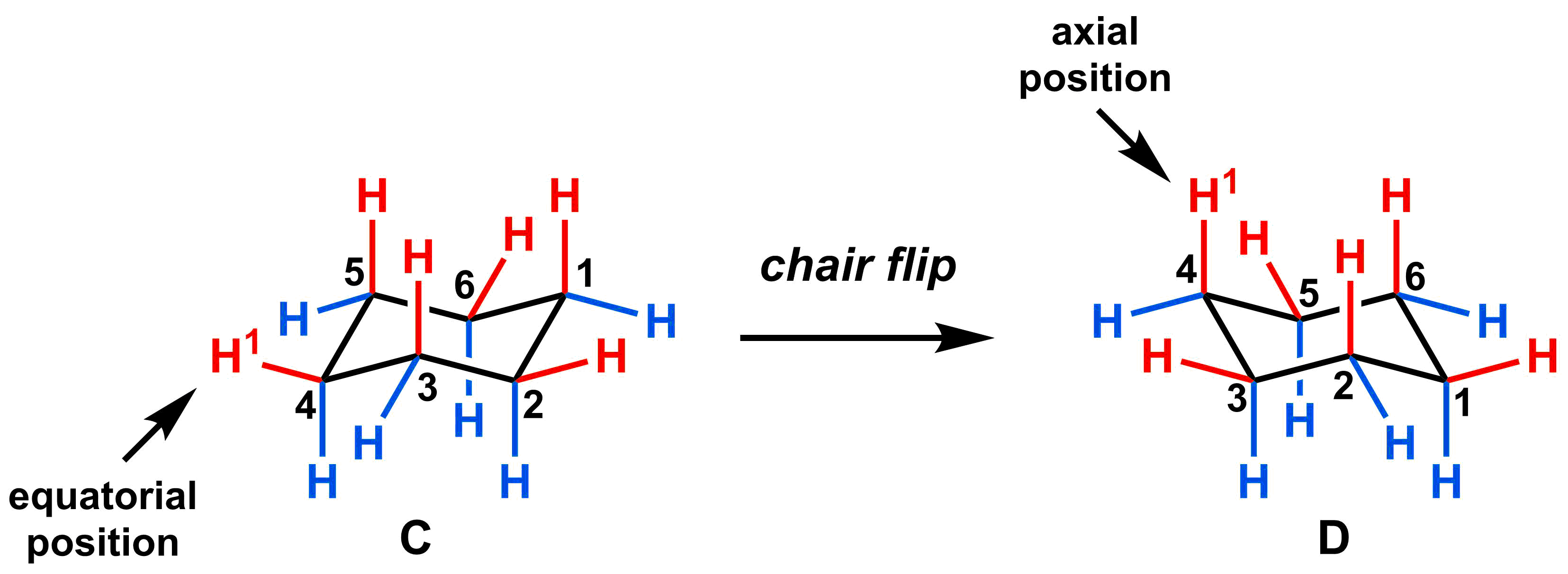
In the example below, one of the hydrogen in C has been labeled (H1, equatorial position). That hydrogen atom would remain in the “up” position after a chair flip, but now takes an axial position as shown in molecule D.
Interactive: