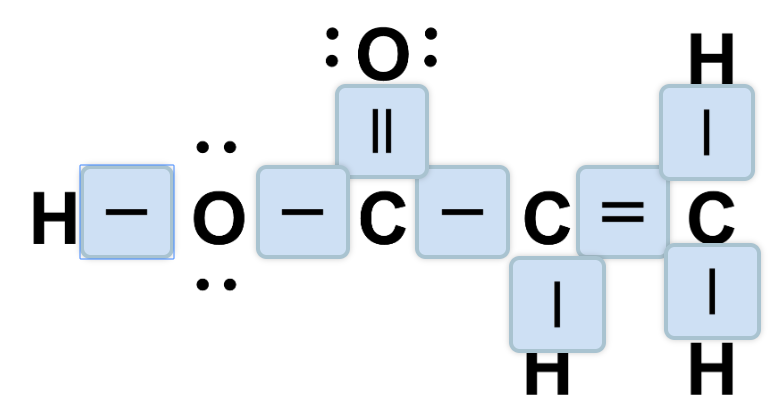
- To simplify structures, two dots representing a bond in a Lewis dot structure can be represented by a line.
To simplify the structure, we can replace each pair of bonding electrons in a Lewis dot structure with a straight line connecting the two bonded atoms. Such diagrams are called Kekule structures, and give the same information as Lewis dot structures: they show the locations of lone pair and bonding electrons, but do not necessarily reflect the geometry of the molecule.
The table below shows the relationship between Lewis dot structures and Kekule structures. All bonding electrons in a Lewis dot structure (shown in red) are converted to bonds in a Kekule structure (also shown in red).

Video removed for updates
Interactive:
View solution (try yourself before checking!):