- More electronegative atoms attract electrons towards themselves more strongly in molecules
- Molecules with an equal distribution of electrons are non-polar, while those with unequal electron distributions are polar
- More electronegative atoms are more stable with negative charges than less electronegative atoms
- Less electronegative atoms are more stable with positive charges than more electronegative atoms
- The electronegativity of an atom changes depending on its hybridization
Electronegativity is a measure of how strongly an atom attracts electrons towards itself in a bond; the higher the electronegativity, the more the atom attracts electrons to itself. Electronegativity generally increases across the periodic table from left to right and from bottom to top, so that fluorine is the most electronegative atom. Below is a periodic table with the Pauling electronegativity included for each atom.

If two bonded atoms have similar electronegativities, they share the bonding electrons equally. We can visualize how electrons are shared using electrostatic potential maps (EPMs). EPMs visually represent the electron density across a molecule. In an EPM, red indicates negatively charged regions of the molecule (i.e. a high electron density), green indicates neutral regions of the molecule, and blue indicates positively charged regions of the molecule (i.e. a low electron density).

Let’s consider diatomic oxygen, O2. The two bonded atoms are both oxygen, so they have the same electronegativity (3.44 on the Pauling scale). The electrons are shared equally, so we wouldn’t expect any areas of high (red) or low (blue) electron density. We can see this in the EPM of O2 below, which is all green (i.e. neutral). What happens if our bonded atoms had different electronegativities? Consider hydrogen fluoride, HF: hydrogen has an electronegativity of 2.20 and fluorine has an electronegativity of 3.98. Fluorine’s higher electronegativity means that it pulls electrons towards itself more strongly than hydrogen. Thus, we’d expect high electron density near fluorine (red) and a corresponding low electron density near hydrogen (blue). This is exactly what we see in the EPM of HF, below.

When two bonded atoms have a small electronegativity difference (approximately 0.4 or less), they essentially share the bonding electrons equally and we call the bond non-polar covalent. If the electronegativity difference between the two atoms is large (approximately 1.7 or greater), the bond is generally classified as ionic because the molecule is essentially fully ionized. If the electronegativity difference between the bonded atoms is between these two values (approximately 0.4 and 1.7), one of the atoms has a significantly greater share of the bonding electrons and we call the bond polar covalent because there are two different charged regions or “poles”. Note that bonding is a continuum, with non-polar covalent at one extreme and pure ionic on the other extreme; the numbers given here are guidelines only, and may differ slightly in other textbooks.

If a molecule has polar bonds that are arranged asymmetrically around the molecule, the overall molecule will have a permanent dipole. That is, one side of the molecule will have a slightly positive charge (blue), and the other side will have a slightly negative charge (red). For example, consider dichloromethane, CH2Cl2. Carbon has an electronegativity of 2.55, hydrogen has an electronegativity of 2.20, and chlorine has an electronegativity of 3.16. The C-H bonds have an electronegativity difference of 0.35, so they are non-polar covalent. The C-Cl bonds have an electronegativity difference of 0.61, so they are polar covalent, with a higher electron density near Cl. Since the polar C-Cl bonds are not arranged symmetrically (that is, the bond dipoles don’t cancel each other out), the molecule will have a higher concentration of negative charge on one side than the other. To indicate a dipole we draw an arrow with the “+” closer to the partially positively charged side of the molecule and an arrow closer to the partially negatively charged side.

Now consider tetrachloromethane, CCl4. Each C-Cl bond is still polar, but the individual dipoles of the bonds cancel out because the bonds to Cl are arranged symmetrically. The result is no net dipole, as we can see in the EPM, below.

Why do we care about electron distributions?
Electron distributions are important in predicting properties of molecules and chemical reactions. For example, molecules that are polar (have permanent dipoles) dissolve better in solvents that are also polar, while molecules that are non-polar (don’t have a permanent dipole) dissolve better in solvents that are also non-polar. Such considerations are important in applications such as drug design, where the polarity of a molecule affects which part of the body the drug targets. In chemical reactions, the atoms in a molecule that have a partial negative charge often react with the atoms in another molecule that have a partial positive charge. Thus, knowing how the electrons are distributed in molecules can help us predict how they will react.
So far we’ve focused on molecules that have no net charge. Now, we’ll consider charged molecules. We’ll see that the electronegativity of the atom that bears the positive or negative charge in a molecule has a big effect on the molecular stability.
For example, consider the two anions below: one bears a negative charge on oxygen, while the other bears a negative charge on nitrogen. The molecule with the negative charge on the more electronegative atom (oxygen) is more stable than the one with the negative charge on the less electronegative atom (nitrogen). This is true only for atoms in the same row of the periodic table, more electronegative atoms are better at stabilizing negative charges.

Why are more electronegative atoms better at stabilizing negative charge?
A full understanding of why more electronegative atoms are better at stabilizing negative charges would require us to delve deeper into quantum chemistry and molecular orbital theory, but we can get an intuitive understanding using what we’ve studied so far. If you look back at the previous section where we studied orbitals, you may notice that the orbitals of more electronegative atoms tend to be lower in energy. If we have an excess of electrons (i.e. a negative charge), they will be the most stable in the lowest energy orbitals. Since more electronegative atoms tend to have the lower energy orbitals, negative charges tend to be more stable on more electronegative atoms.
The opposite is true for positively charged molecules. Since highly electronegative atoms hold onto their electrons tightly, it is difficult to remove an electron from an electronegative atom and the resulting cation is not very stable. Thus, less electronegative atoms are better at stabilizing positive charges. For example, consider the two molecules shown below.
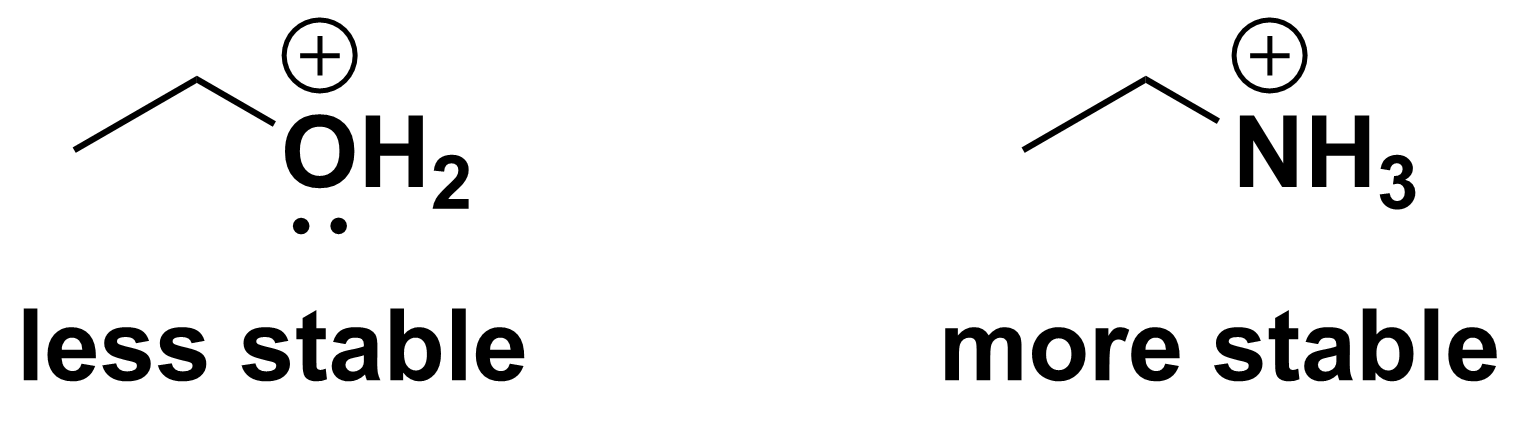
The molecule on the left has a positive charge on oxygen, while the molecule on the right has a positive charge on nitrogen. Since nitrogen is less electronegative than oxygen, it is better able to stabilize the positive charge. Thus, the molecule on the right is more stable than that on the left.
Electronegativity and hybridization
The electronegativity of a particular atom can shift depending on its hybridization. More electronegative atoms tend to hold their electrons more closely in lower energy orbitals. In organic chemistry, we focus on three different hybrid orbitals: sp, sp2, and sp3. Since s-orbitals are lower in energy and held closer to the nucleus than p-orbitals, the more s-character a hybrid orbital has (i.e. the higher the percent contribution of s to the overall makeup of the hybrid orbital), the more electronegative it is.

That is, given three atoms of a particular element with sp vs sp2 vs sp3 hybridization, the atom with sp hybridization will be the most electronegative (largest s-character) and the atom with sp3 hybridization will be the least electronegative (smallest s-character).
Interactive: