How are pharmaceuticals made? How are polymers produced? The answer to both of these questions is through the use of chemical reactions. Chemical reactions are processes in which one molecule can be converted to another. This may involve a process to change the oxidation state of the molecule, or it may involve the construction of new covalent bonds. Unlike uncontrolled reactions, such as combustion, chemical reactions aimed at building new molecules are controlled and typically transform only one portion of the molecule, usually at a specific functional group. Through careful selection of starting materials and reaction conditions, many useful products can be made in the laboratory, ranging in complexity from nylon to life-saving, anti-cancer medications.
This section will focus on one of the simplest chemical reactions, second order nucleophilic substitution (SN2) reactions. This reaction class is frequently observed in biological processes, such as the conversion of norepinephrine to epinephrine (i.e. adrenaline). Such reactions are also commonly used in chemical laboratories to construct new covalent bonds and can help explain how some pesticides and chemical warfare agents work. In the context of this course, studying SN2 reactions utilizes information from every aspect of what we have covered so far, including kinetics, bonding theories, sterics, electronics, and stereochemistry.
The last step of the biosynthesis of epinephrine (i.e. adrenaline) involves the transfer of a methyl group from S-adenosylmethionine (SAM) to norepinephrine via an SN2 reaction. In this reaction, the right portion of SAM (shown in grey) is substituted for norepinephrine. The products are epinephrine and S-adenosylhomocysteine (SAH), as shown below.
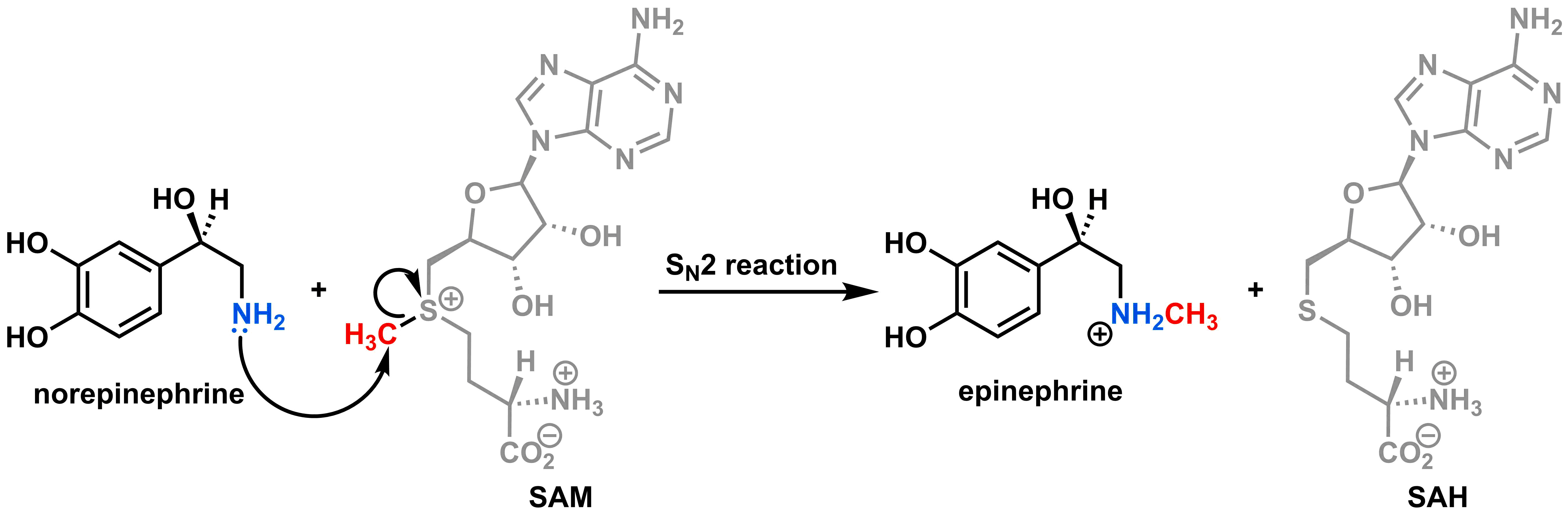
SAM is used in many organism as a way to transfer methyl groups to molecules during biosynthesis.
Methyl halides such as iodomethane (common name: methyl iodide) have been used as pesticides to protect crops such as strawberries. Iodomethane achieves the same effect synthetically that SAM does biologically (see above): it adds a methyl group to a molecule. When used as a pesticide, iodomethane can add methyl groups to the DNA of insects, thus damaging the DNA and killing the bugs. Let's look at how the substitution reaction works, below:
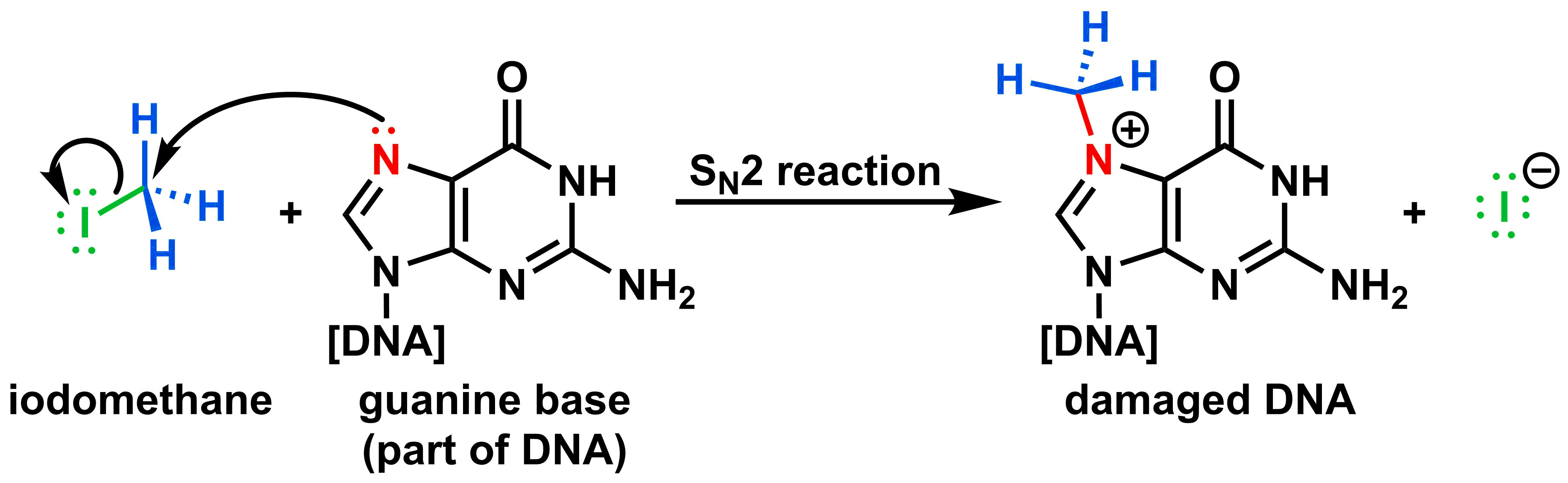
The I (shown in green) attached to the methyl group (show in blue) is substituted with a reactive nitrogen (shown in red) in DNA. The addition of a methyl group disrupts DNA replication, causing mutations and/or cell death.
Of course iodomethane does not differentiate between the DNA of insects and other animals (including us!). Thus, using it as a pesticide is concerning, since it is toxic and likely carcinogenic to humans. Protests from scientists and citizens halted the use of iodomethane as a pesticide in California in 2012.
Mustard gas has been used as a chemical weapon since World War I. A similar compound known as mustine, which replaces the S in mustard gas with an NH, was used as the first chemotherapy drug. Both chemicals act via the same mechanism: a series of substitution reactions damage DNA, which prevents cell division and generally leads to apoptosis (i.e. programmed cell death). Let's look at how the DNA is damaged:

In the first step, mustard gas undergoes an intramolecular substitution reaction. Intramolecular means that the reaction takes place between different parts of the same molecule, rather than between two (or more) separate molecules. At the carbon circled in red, the Cl is substituted for the S, which gives the blue sulfonium ion as the product.
This blue sulfonium ion is highly reactive, and undergoes another substitution reaction with a nitrogen atom on a guanine base in DNA. In this step, the bond from the reactive carbon (circled in green) to S is substituted for a bond to the reactive N on guanine (highlighted in red). When the blue group is bonded to DNA, the DNA can no longer replicate and the cell is destroyed.