- When an SN2 reaction occurs at an asymmetric carbon on the electrophile, the product's stereochemistry is inverted relative to the reactant.
- The stereochemistry only inverts at the electrophile's reactive carbon, not at remote stereocentres.
Up until now, most of our example SN2 reactions have been on achiral electrophiles. If we use an electrophile where the reactive carbon is an asymmetric centre, backside attack results in a relative inversion of stereochemistry. To see why, consider the generic SN2 reaction below.
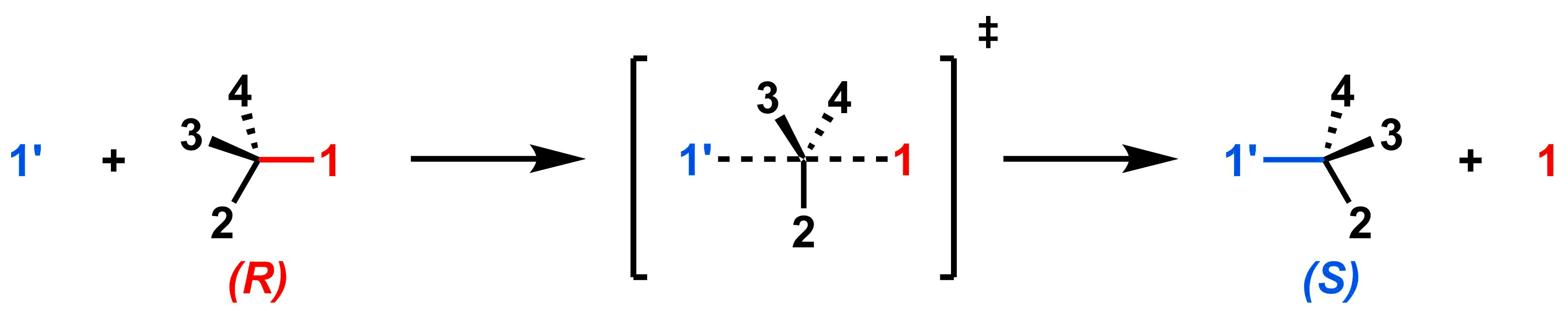
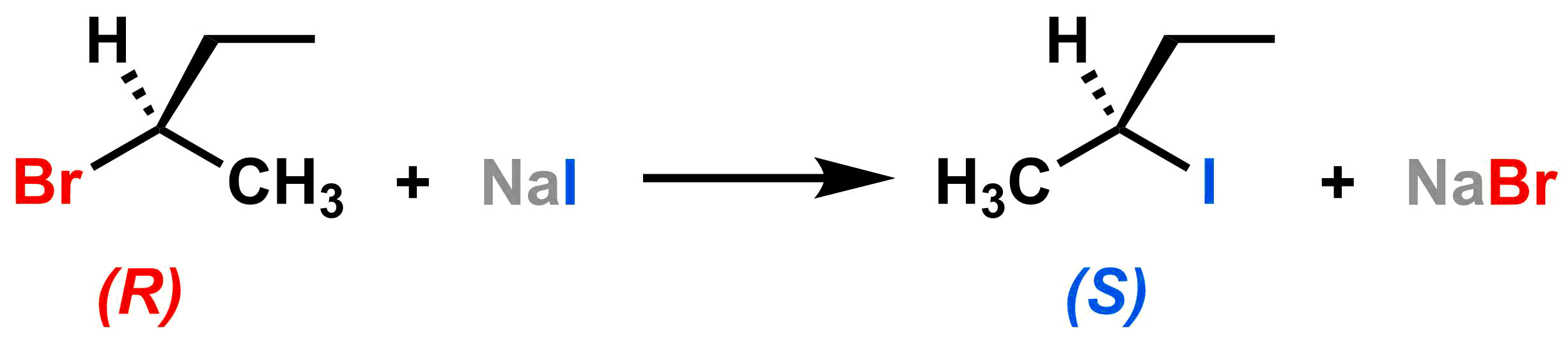
The numbers represent the relative CIP priorities of the substituents, so our reactant has (R) configuration. As the red leaving group (priority #1 in the reactant) leaves, the blue nucleophile (priority #1 in the product) approaches via backside attack, and the three other substituents "swing" from the left side to the right side. With these priorities, the configuration of the product is (S); that is, it is inverted relative to the reactant. The example below shows how (R)-2-bromobutane can be converted into (S)-2-iodobutane through an SN2 reaction with NaI.
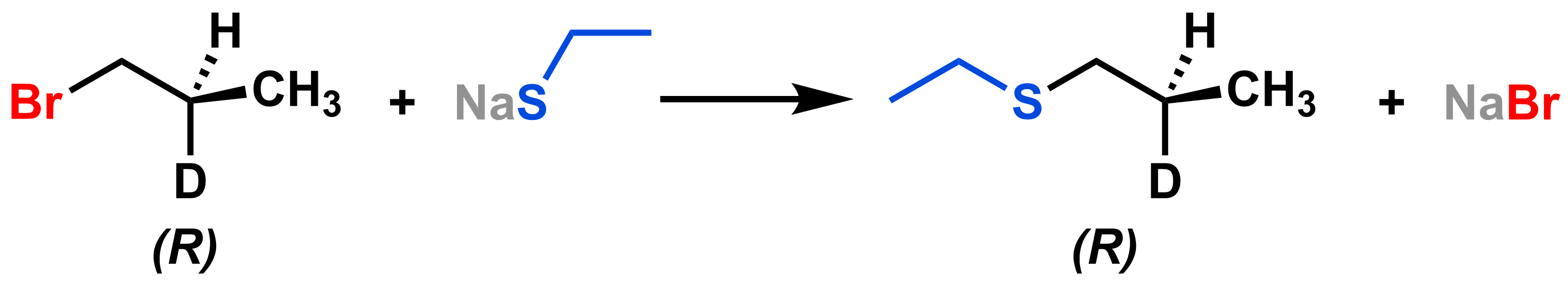
The stereochemistry only inverts at the reactive carbon, so if the electrophile has an asymmetric carbon elsewhere, like the asymmetric carbon with (R) configuration in the molecule below, it will not be inverted.
Guided:
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
Interactive: