- Reaction schemes aren't always written as balanced reactions; byproducts are sometimes left out to highlight the desired organic product.
- In a substitution reaction, one part of a molecule (the leaving group) is substituted for a new part (the nucleophile).
- Substitution reactions involve an electrophile (accepts electrons), a nucleophile (donates electrons) and a leaving group (the part of the electrophile that is replaced). In the desired organic product, there is a new bond between the electrophile and nucleophile.
Drawing Reaction Schemes:
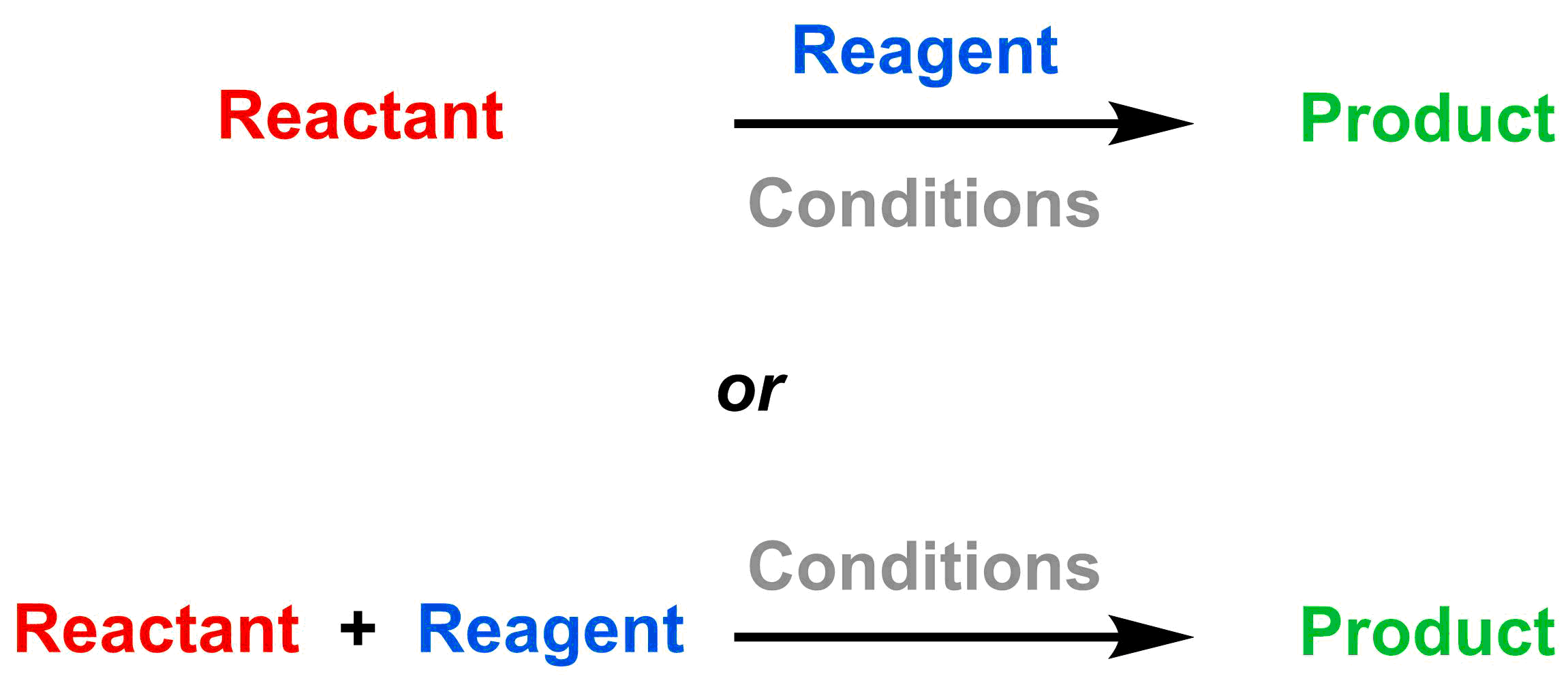
The goal of a chemical reaction is to convert a molecule (the reactant) into a new molecule (the product). To enable the chemical reaction, reactive molecules (the reagents) must be added. By convention, in a reaction scheme the reactant(s) are always listed on the left side of the arrow and the product(s) are listed on the right side of the arrow. The reagents are listed either (1) above the arrow, or (2) on the left side of the arrow. Other reaction conditions, such as the reaction medium (solvent) and temperature, are usually listed either (1) below the arrow if the reagent is included over the arrow, or (2) over the arrow if the reagent(s) are listed next to the reactant. These two general reaction schemes are shown below.
Substitution Reactions:
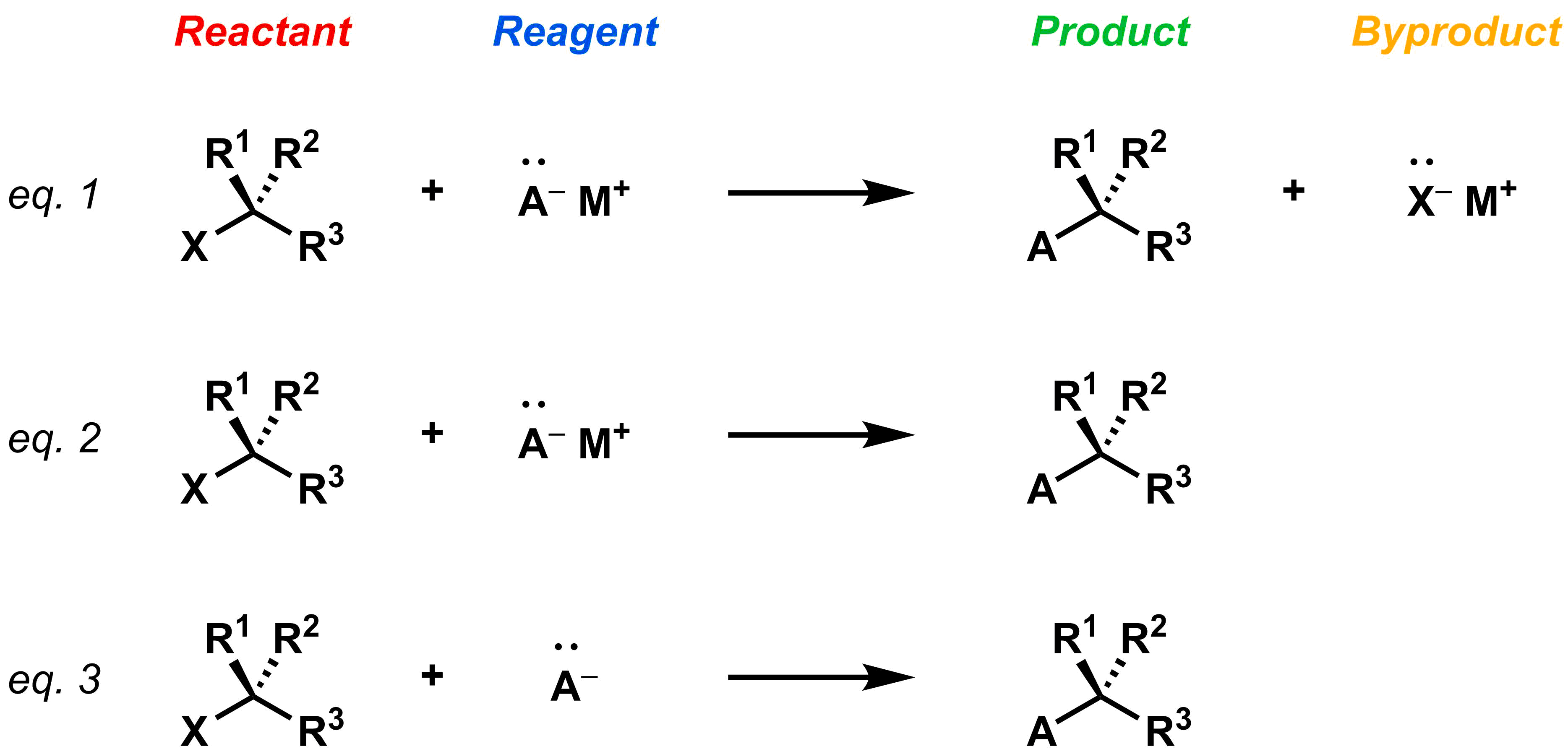
This section will focus exclusively on substitution reactions, in which one part of a molecule is substituted for a new part. For example, in the generic substitution reaction below, the X in the reactant is substituted for the A in the desired organic product. In this case, A-M+ is the reagent and X-M+ is the reaction byproduct (i.e. not the desired organic product of the reaction). After a reaction is carried out in a laboratory, the product is isolated from all other reaction components (unreacted reagents and reactants) as well as any byproducts (eq 1). Because the byproducts are not the goal of the chemical transformation and are usually discarded after a successful reaction, they are often omitted in reaction schemes (eq 2). In introductory chemistry courses, the metal counter ion (M+) is also often omitted in the reaction scheme (eq 3). The scheme below shows three acceptable representations of the same chemical reaction.
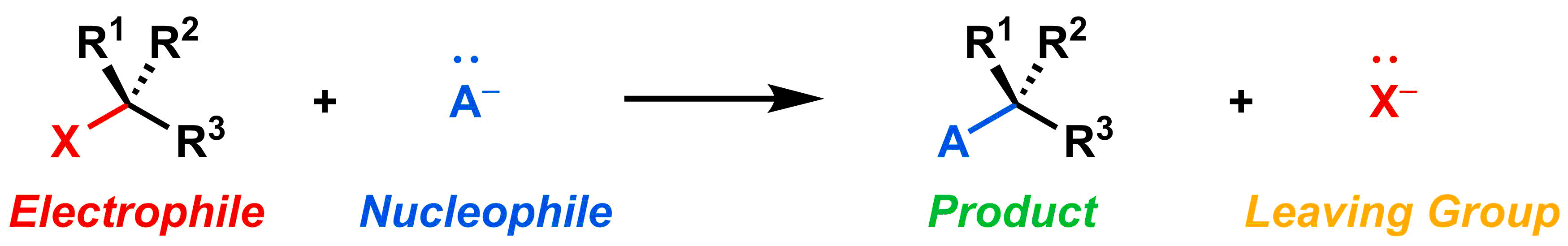
There are four key components in every substitution reaction: (1) the leaving group (2) the nucleophile, (3) the electrophile, and (4) the desired organic product.
(1) Leaving group
The leaving group is the part of the molecule that leaves/is replaced. When it leaves, it takes the two electrons from the bond (shown in red above) with it, so it must be stable with these extra electrons. In the generic reaction above, the X is the leaving group. On the reactant side it is attached to the electrophile, and on the product side it has left as X- and is a reaction byproduct.
Try it: Select the leaving group in the reaction below:
(2) Nucleophile
Nucleo- means nucleus, and -phile is latin for lover, so the nucleophile is a "nucleus lover" because it is attracted to positive charges (such as the nucleus). The nucleophile has a lone pair of electrons available to share, usually with a partial or full negative charge. It shares this lone pair of electrons with the electrophile to form the new bond in the product. In the reaction above, the A- is the nucleophile, since it donates the electrons to form the new blue bond in the product.
Try it: Select the nucleophile in the reaction below:
(3) Electrophile
Electro- means electron, and -phile is latin for lover, so the electrophile is an "electron lover" because it is attracted to negative charges (such as electrons). Electrophiles tend to have full (or partial) positive charges, and are thus attracted to areas of high electron density (i.e. negative charges). In a substitution reaction, the electrophile accepts a lone pair of electrons from the nucleophile, which forms the new bond between the nucleophile and electrophile. In the reaction above, the electrophile is the black reactant on the left, since it accepts electrons from A-.
Try it: Select the electrophile in the reaction below:
(4) Desired organic product
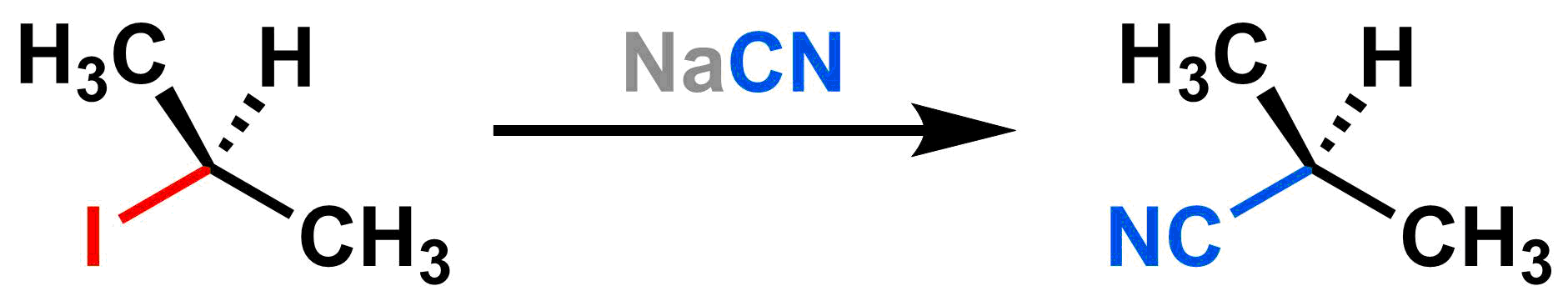
The desired organic product of a substitution reaction is the molecule that has a covalent bond between the electrophile and the nucleophile. Sometimes you will see only this product written in a substitution reaction and the leaving group byproduct will be omitted. An example using this convention is shown below:
Interactive: