- SN1 reactions occur over two elementary steps: (1) the leaving group leaves to form a carbocation, then (2) the nucleophile reacts with the carbocation.
- When an SN1 reaction occurs at an asymmetric carbon on the electrophile, the products with inverted and retained relative stereochemistry are both produced.
- The first step (when the leaving group leaves) is the rate determining step
Mechanism and curved arrows:
Just as you have learned with SN2 reactions, there are four key components in every SN1 reaction: (1) the electrophile, (2) the nucleophile, (3) the leaving group, and (4) the desired organic product.
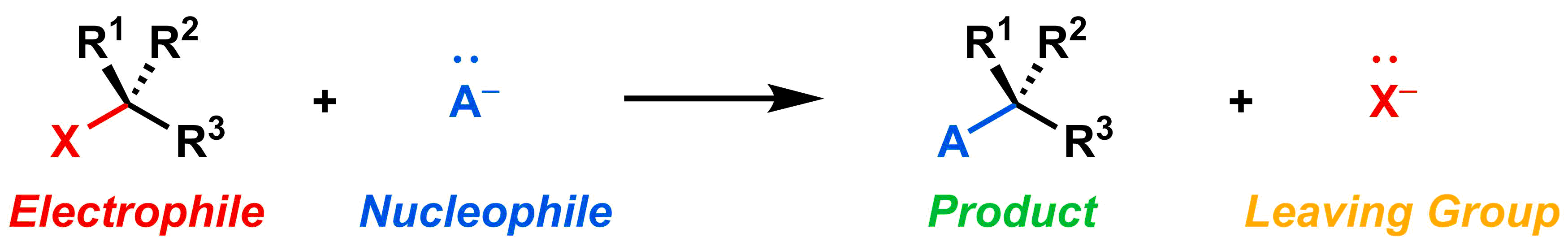
Reaction Mechanism
As you can see below, the overall processes of an SN1 and SN2 reaction are almost identical.

The key difference is that an SN2 mechanism gives only the product with relatively inverted stereochemistry, while the SN1 mechanism gives products with both inverted and retained stereochemistry. This difference in stereochemistry arises from the fundamental difference in the mechanism of these two reactions. Recall that for an SN2 reaction, the process is concerted (i.e. bond forming and bond breaking occur during the same step), so only one elementary reaction makes up the overall process. In an SN1 reaction, the same general overall reaction occurs, but the bond forming and bond breaking are not concerted and instead occur over two elementary steps:
Step 1: The bond between the leaving group and the electrophilic centre breaks.
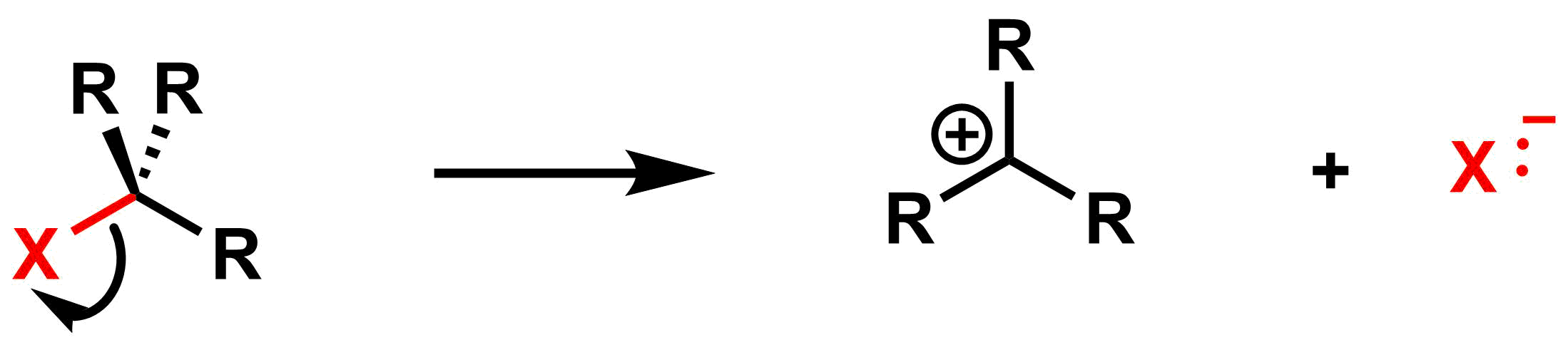
Step 2: A bond forms between the nucleophile and the electrophilic centre.
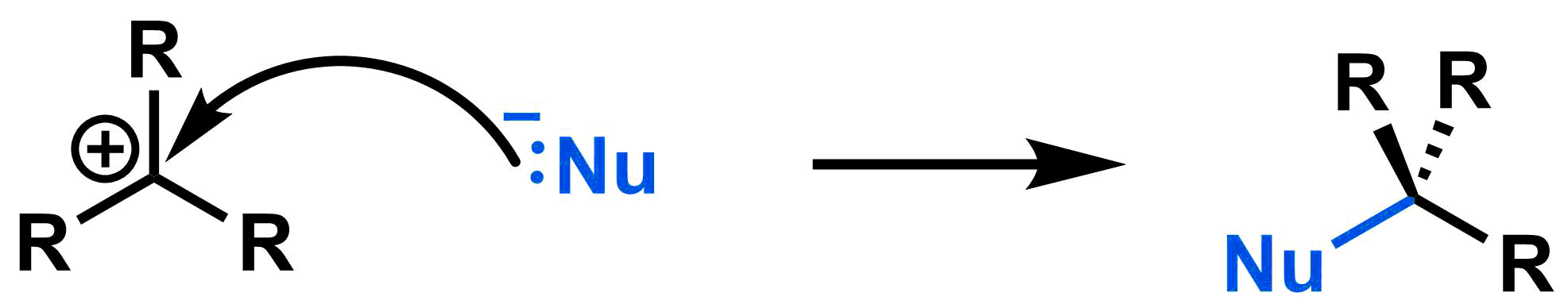
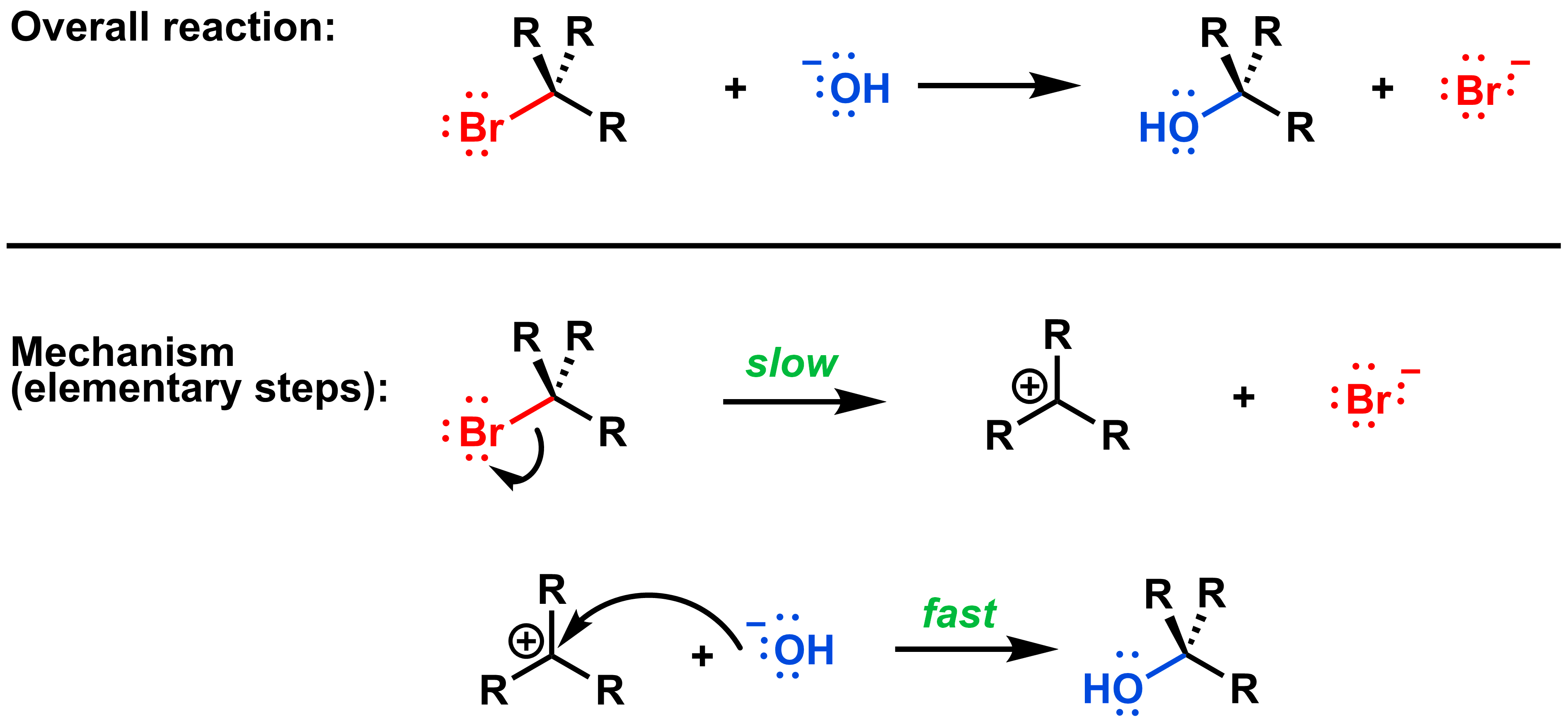
All SN1 mechanisms have these two fundamental steps: first the leaving group leaves, then the nucleophile attacks the electrophilic carbon. If the nucleophile is anionic, these two steps make up the entire reaction mechanism, as shown below:
Sometimes, you may see an additional mechanistic step before and/or after the two steps in the core SN1 mechanism. For example, if the nucleophile is neutral, then a final acid/base step after the two SN1 steps is necessary to give a neutral product, as shown below. The nucleophile (and/or reaction medium, i.e., solvent) is usually used as the base in this final step.
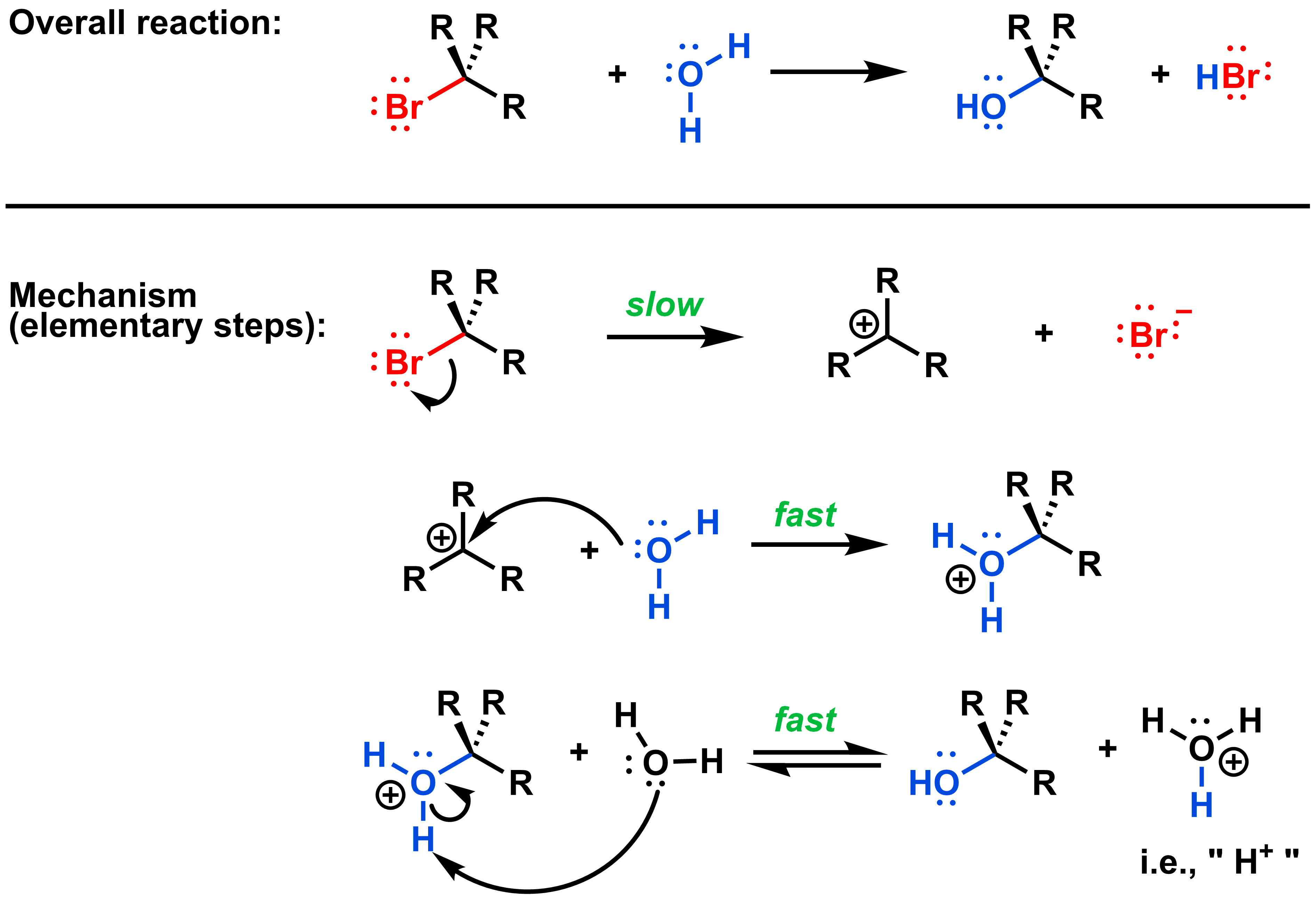
We still soon study another variety of the SN1 reaction, the acid-catalyzed mechanism, which involves acid/base steps both before and after the two core SN1 steps.
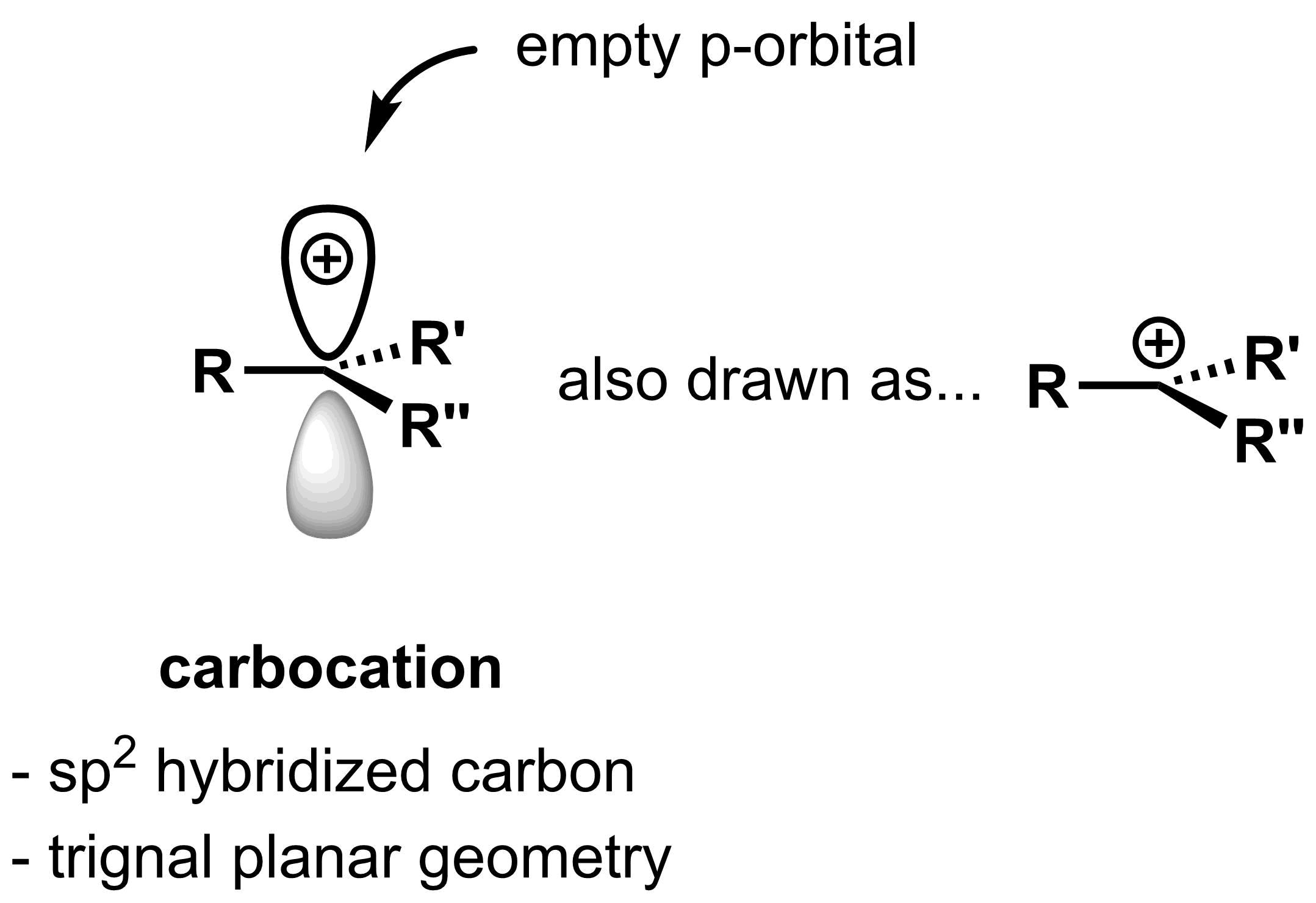
Carbocation
This reaction mechanism introduces a new species: the carbocation. The carbocations is an intermediate. Carbocations are typically very reactive as they do not possess a full octet. The carbon atom bearing the formal positive charge is sp2 hybridized, so carbocations have a trigonal planar geometry with an empty p-orbital. While the positive charge is indicated in only one of the lobes, the charge is evenly distributed among both lobes. The p-orbital is indicated for clarity and may be omitted when drawing carbocations.
A carbocation is the intermediate after the first step of an SN1 mechanism, when the leaving group leaves. This intermediate is consumed in the second step when it reacts with the nucleophile. Since this intermediate is planar, the nucleophile can approach the electrophile from either the top or the bottom face.
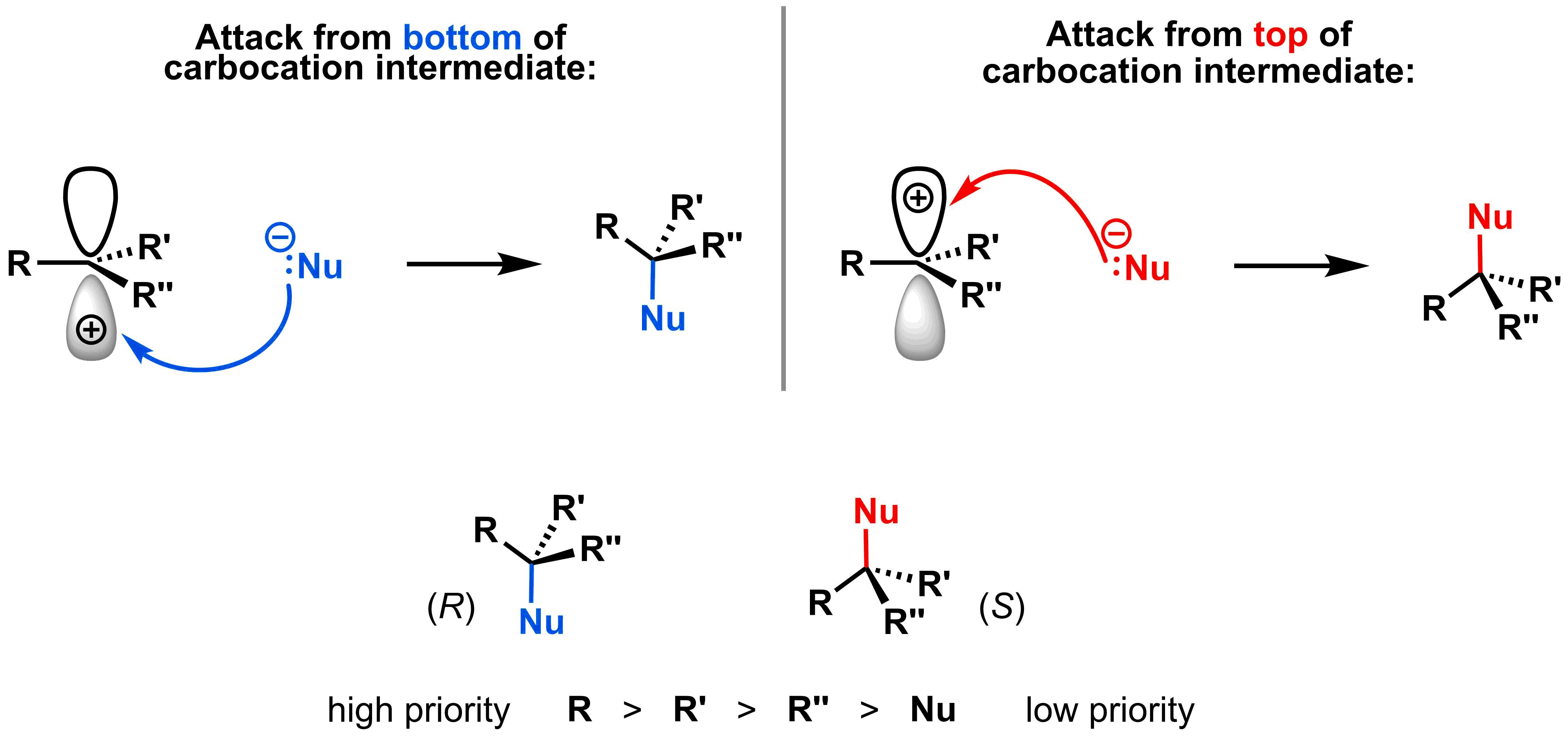
As a result, if the electrophilic carbon is an asymmetric centre, then the products with both (R) and (S) configurations will be formed. While there are many exceptions, for this class you can assume that the (R) and (S) configurations will be formed in equal amounts.
Kinetics
The two elementary steps in an SN1 reaction necessitate an intermediate and two transition states. There are two key aspects that still need to be considered: (1) what is the relative energy level of the intermediate compared to the starting material and product, and (2) which step is rate limiting (i.e. the slow step).
First, the carbocation intermediate has an incomplete octet, so will be higher in energy than both the reactants and products, as shown in the diagram below.
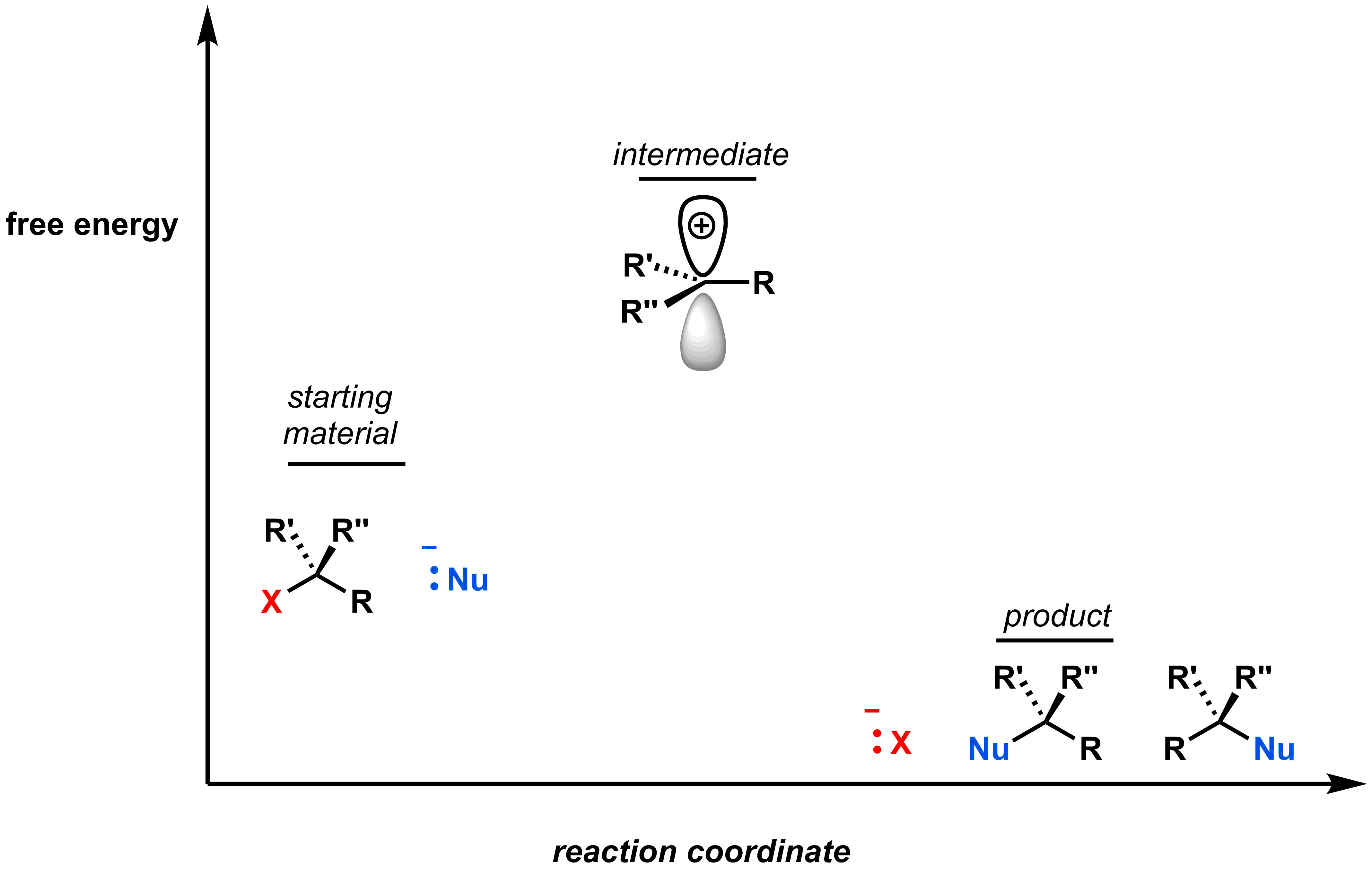
We can determine which step is rate-limiting if we know the relative sizes of the free energy of activation for each step. Recall that in the first step of an SN1 reaction, the leaving group leaves forming an anionic leaving group and a carbocation. This requires a large free energy of activation to access the high-energy intermediate. Once formed, the free energy of activation to return to a stable/neutral product is low. This is depicted in the reaction coordinate diagram: the barrier (i.e. free energy of activation) between the reactant and intermediate is higher than between the intermediate and the product.
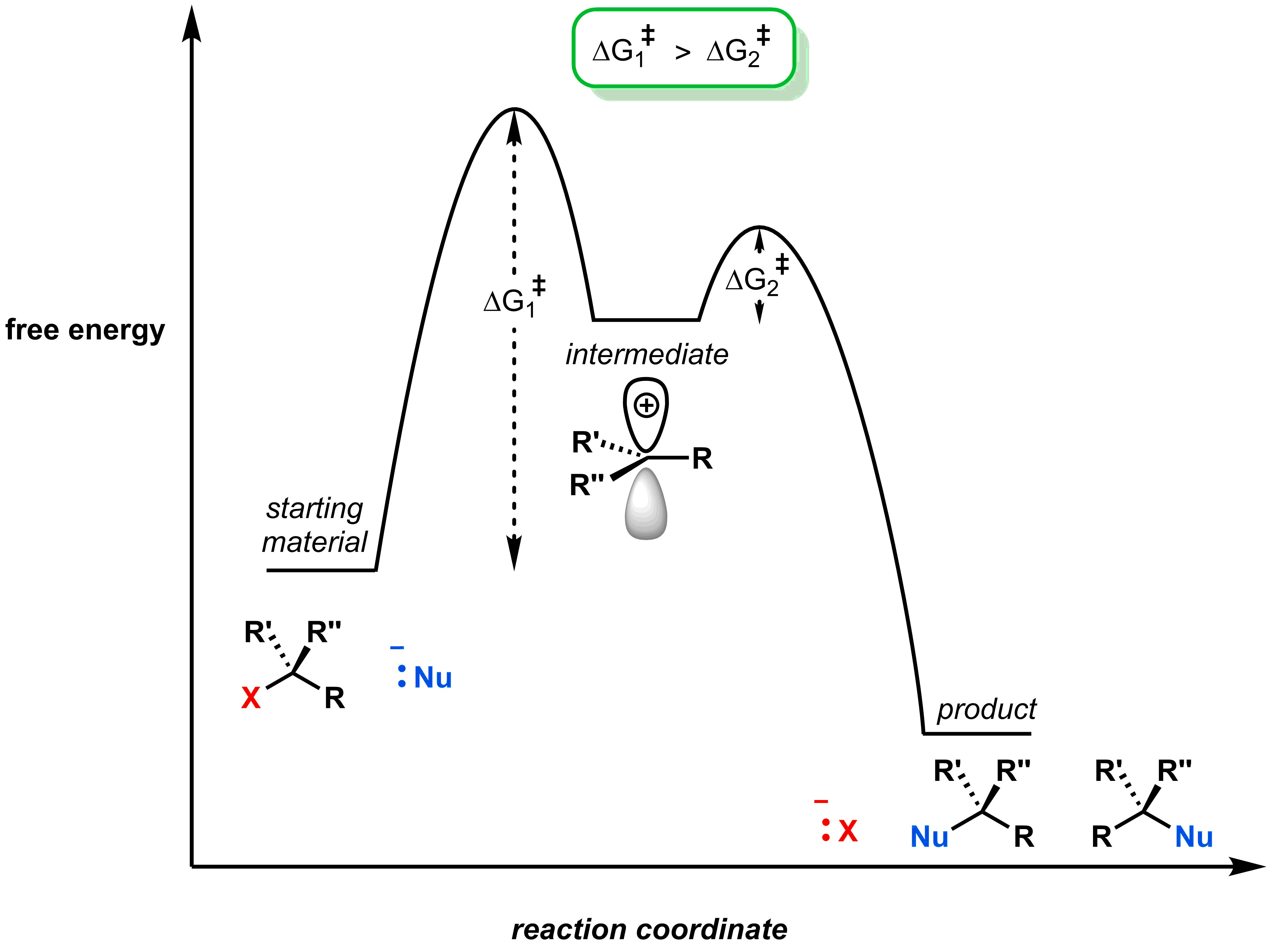
Knowing that the first step is rate determining, you can determine the kinetics of SN1 reactions using your previous knowledge. Complete the activities below to see how your knowledge of kinetics applies to the SN1 mechanism.
Guided:
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
Interactive: