In an SN1 reaction:
- More substituted electrophiles react more quickly because they lead to more stable carbocation intermediates.
- Anionic and neutral nucleophiles react at similar rates.
- Weaker bases generally make better leaving groups.
Electrophile:
In the first step of an SN1 reaction, the bond between the leaving group and the electrophilic centre breaks to form a carbocation intermediate. The more stable the resulting carbocation, the faster the first step will occur. As a general trend, increasing alkyl substitution adjacent to the carbocation leads to increasingly stable carbocation intermediates.
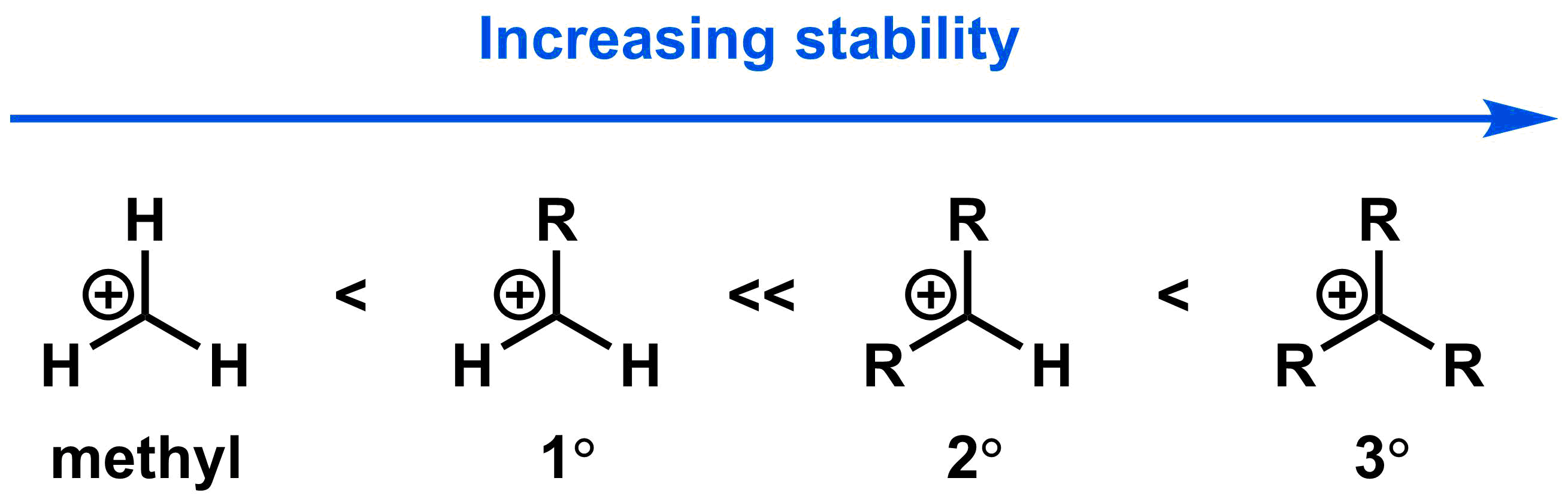
These highly substituted carbocations are more stable because the alkyl groups help minimize the positive charge by donating electron density towards it through hyperconjugation, a special type of orbital interaction that results in distribution of electron density. The details of hyperconjugation are outside of the scope of this course, but will be very important in future chemistry classes. At the most basic level, alkyl groups can be treated as electron-donating substituents; the more alkyl groups surrounding a carbocation, the more the positive charge on the carbocation is stabilized.
Methyl and 1° carbocations are too unstable to readily form under the conditions used for a standard SN1 reaction. As such, methyl and 1° electrophiles will not undergo SN1 reactions. 2° electrophiles lead to 2° carbocations, which are stable enough to undergo SN1 reactions. 3° electrophiles react the most quickly in SN1 reactions because they give the most stable 3° carbocations. The relative rate of the SN1 mechanism between H2O and each electrophile class is shown in the table below; 3° electrophiles react 100,000,000 times more quickly than methyl electrophiles.

Nucleophile:
Nucleophilic attack in an SN1 reaction occurs during the second elementary step, which is after the rate determining step. As a result, it does not appear in the rate law, rate=k[electrophile], and so changing the concentration of the nucleophile does not affect the reaction rate. Any nucleophile with a lone pair (whether negatively charged or neutral) can be used in an SN1 reaction.
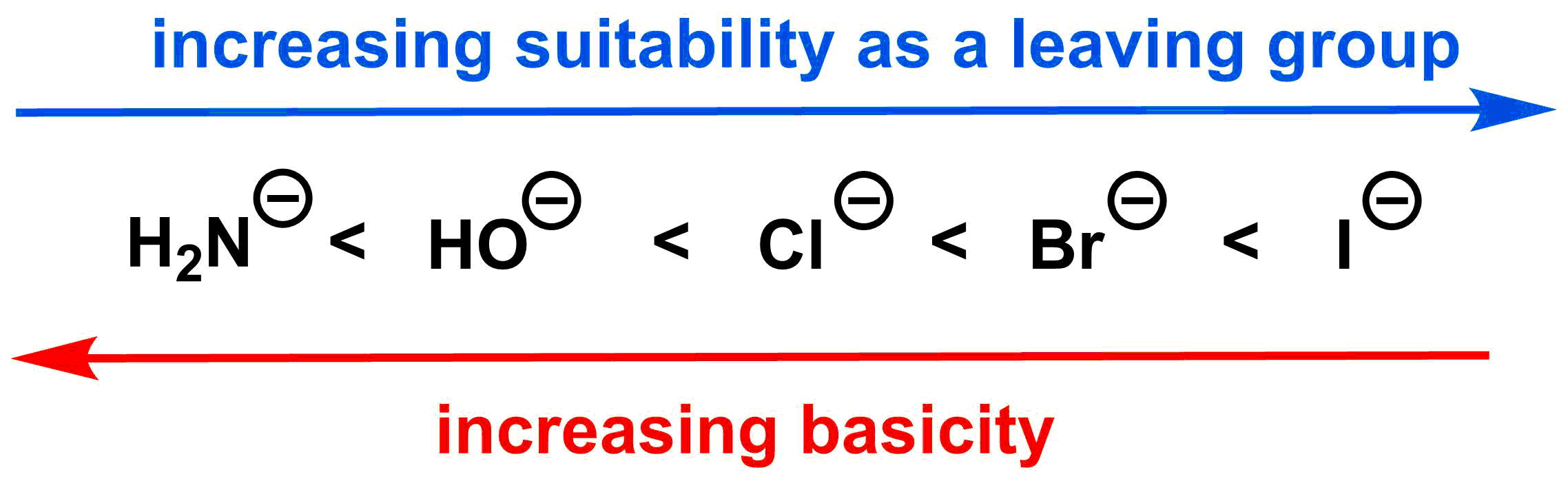
Leaving group:
The leaving group for an SN1 reaction follows the same trend as for an SN2 reaction (see the diagram below).
Guided:
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
Interactive: