- In substitution reactions, methyl and 1° electrophiles react via the SN2 mechanism only, 3° electrophiles react via the SN1 mechanism only.
- 2° electrophiles can react via both the SN1 and SN2 mechanisms. To determine/control which mechanism dominates:
- Anionic nucleophiles favour the SN2 mechanism, while neutral nucleophiles favour the SN1 mechanism.
- High nucleophile concentrations favour the SN2 mechanism, while low nucleophile concentrations favour the SN1 mechanism.
- Polar aprotic solvents favour the SN2 mechanism, while polar protic solvents favour the SN1 mechanism.
- If the product shows relative inversion of stereochemistry (only), then the SN2 mechanism dominated; if both stereoisomers are formed, then the SN1 mechanism was favoured.
Suppose that you want to synthesize the blue molecule shown below.
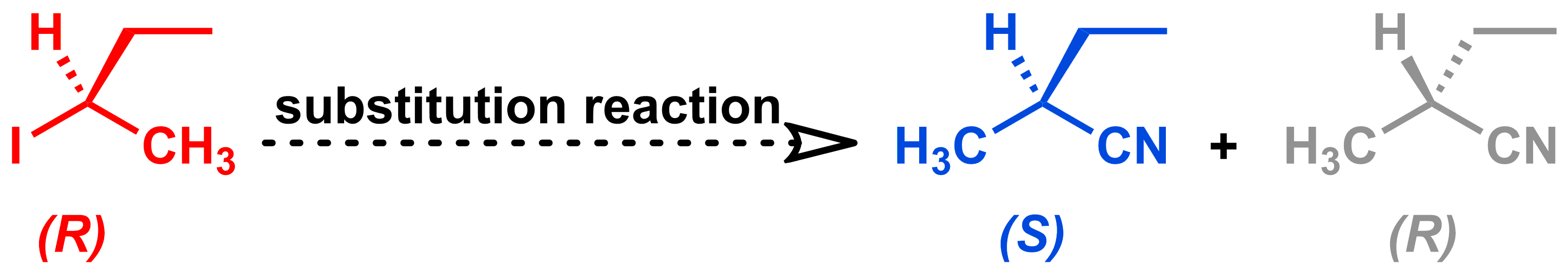
You already have the molecule in red, so plan to carry out a substitution reaction to make your desired molecule. But how can you control if the substitution reaction occurs via the SN1 mechanism (which would give the desired blue product and its grey enantiomer) or the SN2 mechanism (which would give your desired blue product only)? Let's review the differences between these two mechanisms to see what we can do to influence which mechanism occurs.
Electrophile:
Methyl, 1°, and 2° electrophiles can undergo SN2 reactions, while 2° and 3° electrophiles can undergo SN1 reactions. So, if you have a methyl or 1° electrophile undergoing substitution, it must follow the SN2 mechanism. If you have a 3° electrophile undergoing substitution, it must follow the SN1 mechanism. If you have a 2° electrophile, both the SN1 and SN2 mechanisms are possible. Indeed, both these mechanisms will occur simultaneously with 2° electrophiles, but we can adjust the conditions of the reaction to help one mechanism dominate over the other (see below).

Nucleophile:
The nucleophile appears in the rate law of an SN2 reaction, but not of an SN1 reaction. Any changes we make to the nucleophile, then, will change the rate of the SN2 mechanism without affecting the rate of the SN1 mechanism.

Consider first the strength of the nucleophile: using a good (e.g. anionic) nucleophile will speed up the SN2 mechanism without affecting the SN1 mechanism, while using a poor (e.g. neutral) nucleophile will slow down the SN2 mechanism without affecting the SN1. Thus, if you have a 2° electrophile and want the SN2 mechanism to dominate, use a good (e.g. anionic) nucleophile. If you want the SN1 mechanism to dominate, using a poor (e.g. neutral) nucleophile.
Stereochemistry:
If you know the product of your reaction, you can use its stereochemistry to help determine which mechanism must have occurred. If the reactive carbon in the electrophile has inverted stereochemistry (only) relative to the reactant, then the reaction must have occurred via the SN2 mechanism. If the reactive carbon has half (R) and half (S) stereochemistry in the products, the mechanism must have occurred via the SN1 mechanism. If the reactive carbon is not an asymmetric centre, then we don't have any stereochemical information to help us determine which mechanism occurred.
Solvent Classification
Up until now, we have exclusively focused on the reacting partners in substitution reactions. Practically, one often cannot simply combine an electrophile and a nucleophile as (1) the two reagents often mix to form a heterogeneous mixture and, thus, react slowly, and (2) it is difficult to control heat transfer when a reaction contains only the reactive components (i.e. a neat reaction). To address both of these issues, substitution reactions are usually carried out in a liquid reaction medium, called the solvent. Solvents can be divided into three categories:
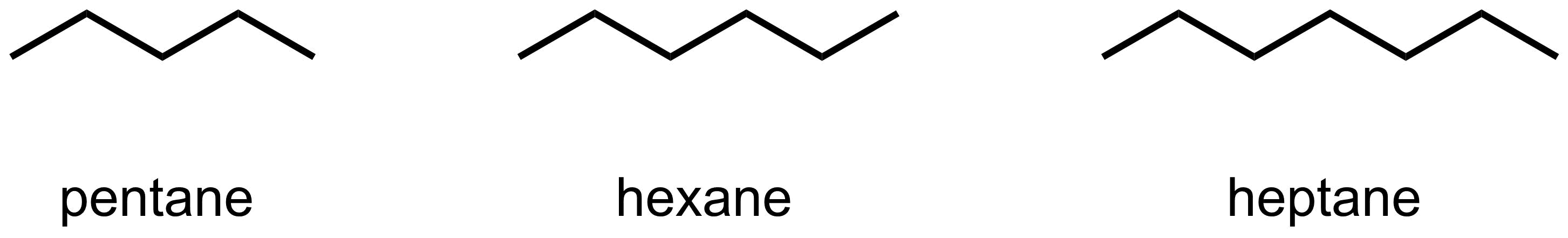
- Non polar solvents: “polar” refers to solvents that have a significant molecular dipole. Therefore, “non polar” solvents do not possess a significant dipole. Most simple hydrocarbon solvents, such as pentane, hexane, and heptane, are non polar.
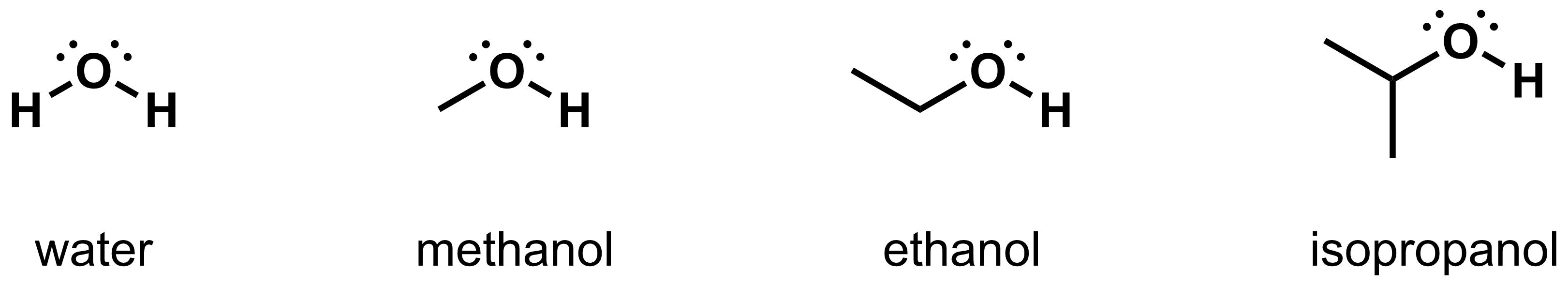
- Polar protic solvents: As the “polar” portion of the name suggests, these solvents contain a significant molecular dipole. “Protic” means that these solvents have O-H or N-H bonds, which are capable of hydrogen bonding. Examples of polar protic solvents are water, methanol, ethanol, and isopropanol.
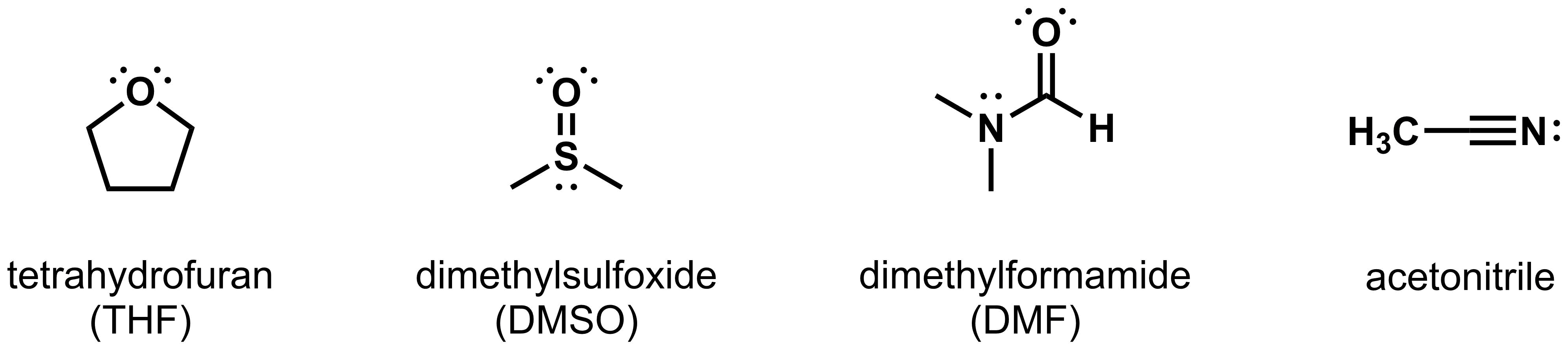
- Polar aprotic solvents: Polar aprotic solvents have a significant molecular dipole, but do not contain any O-H or N-H bonds that are capable of hydrogen bonding. Examples of polar aprotic solvents include tetrahydrofuran (THF), dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF), and acetonitrile.
Choosing a Solvent for Substitution Reactions
For most simple reactions, an appropriate solvent must dissolve both the reactant and reagent. A good rule for remembering which solvent to pick is “like dissolves like”. Thus, charged species (polar) will dissolve more readily in polar solvents and neutral species (non-polar) dissolve more readily in non-polar solvents. All of the substitution reactions presented to date involve charged or partially charged species (e.g. anionic nucleophiles, carbocation intermediates, polar electrophiles). Therefore, these reactions work best with polar solvents.
Polar aprotic solvents for SN2 and SN1 reactions
SN2 reaction proceed most efficiently when polar aprotic solvents are used. Polar aprotic solvents efficiently solubilize the nucleophile and electrophile and stabilize partial positive charges in the transition state through dipole-dipole interactions. For the same reasons, polar aprotic solvents can also be used in SN1 reactions.
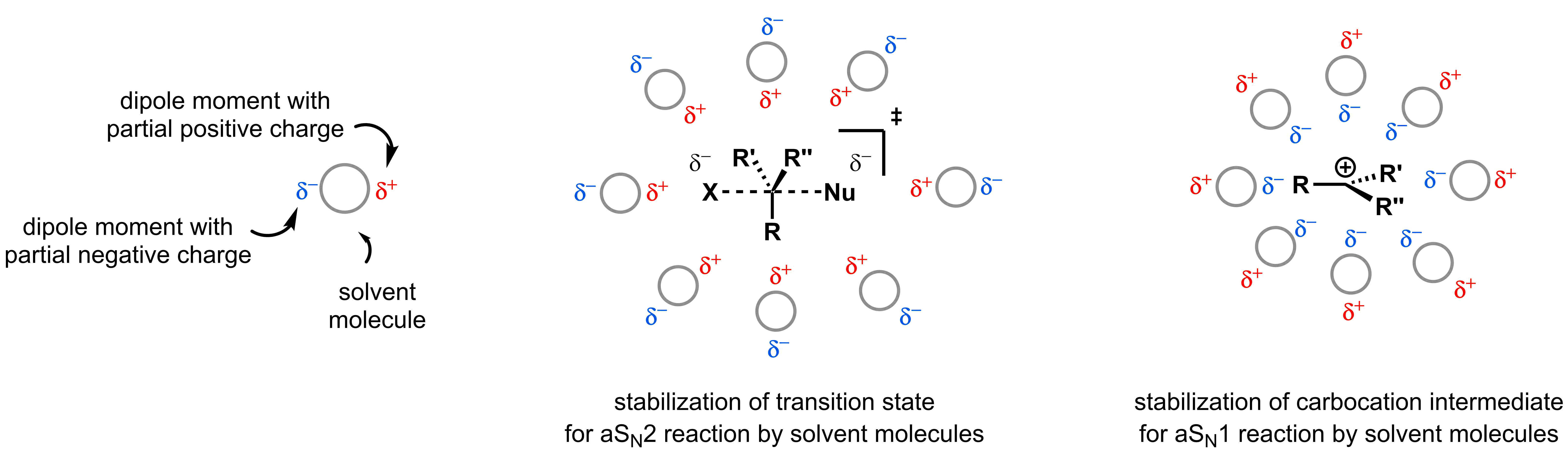
Polar protic solvents for SN2 and SN1 reactions:
While polar protic solvents also solubilize the electrophile and nucleophile in SN2 reactions, they inhibit the reaction for two reasons:
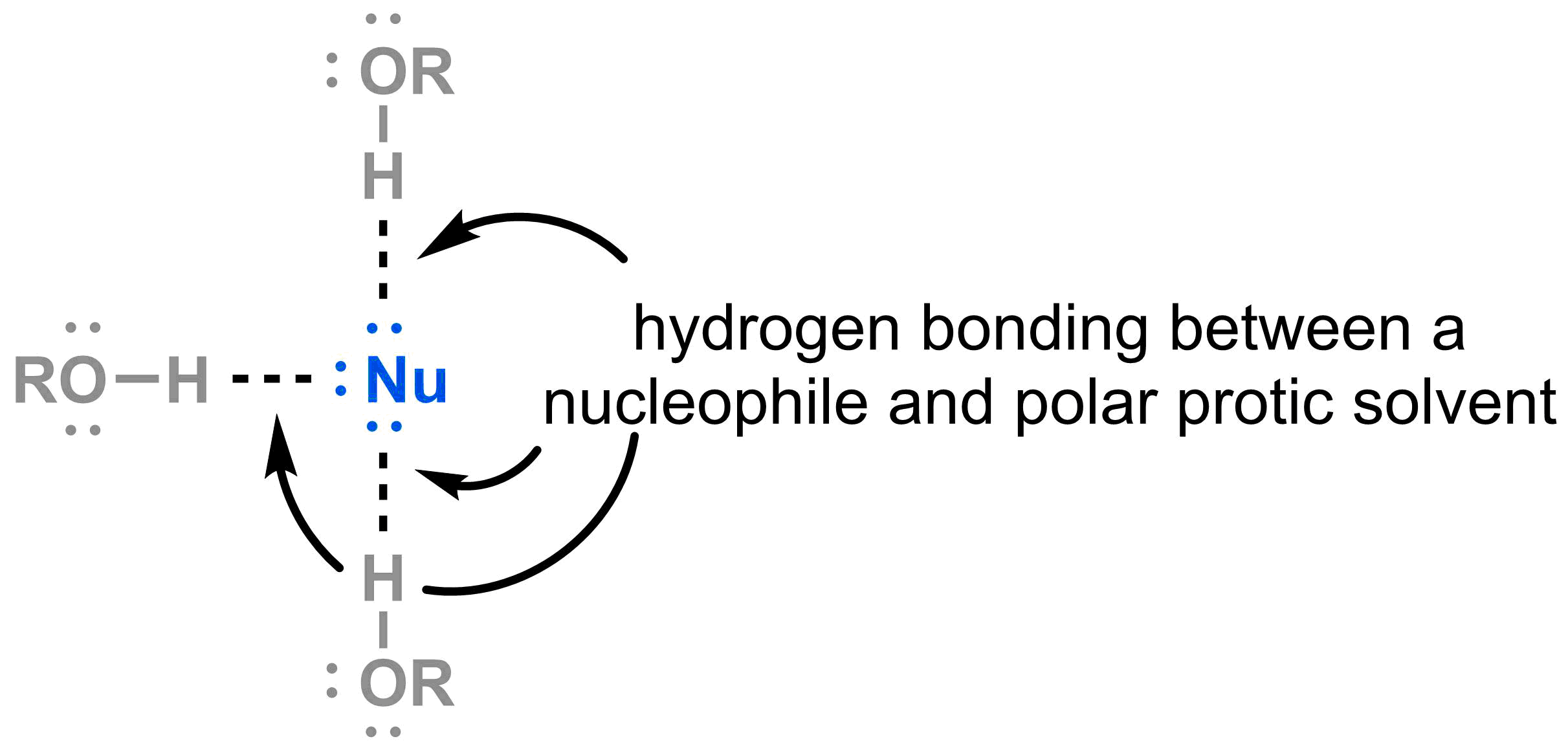
- If the nucleophile is relatively basic, the O-H bond in the solvent protonates it and thus makes it less reactive.
- Even if the nucleophile is not sufficiently basic to be protonated, the lone pair of electrons are capable of accepting a hydrogen bond from the solvent. This results in nucleophiles that are surrounded by a tight shell of solvent molecules, which makes the nucleophile effectively more bulky and slows the rate of the SN2 reaction.
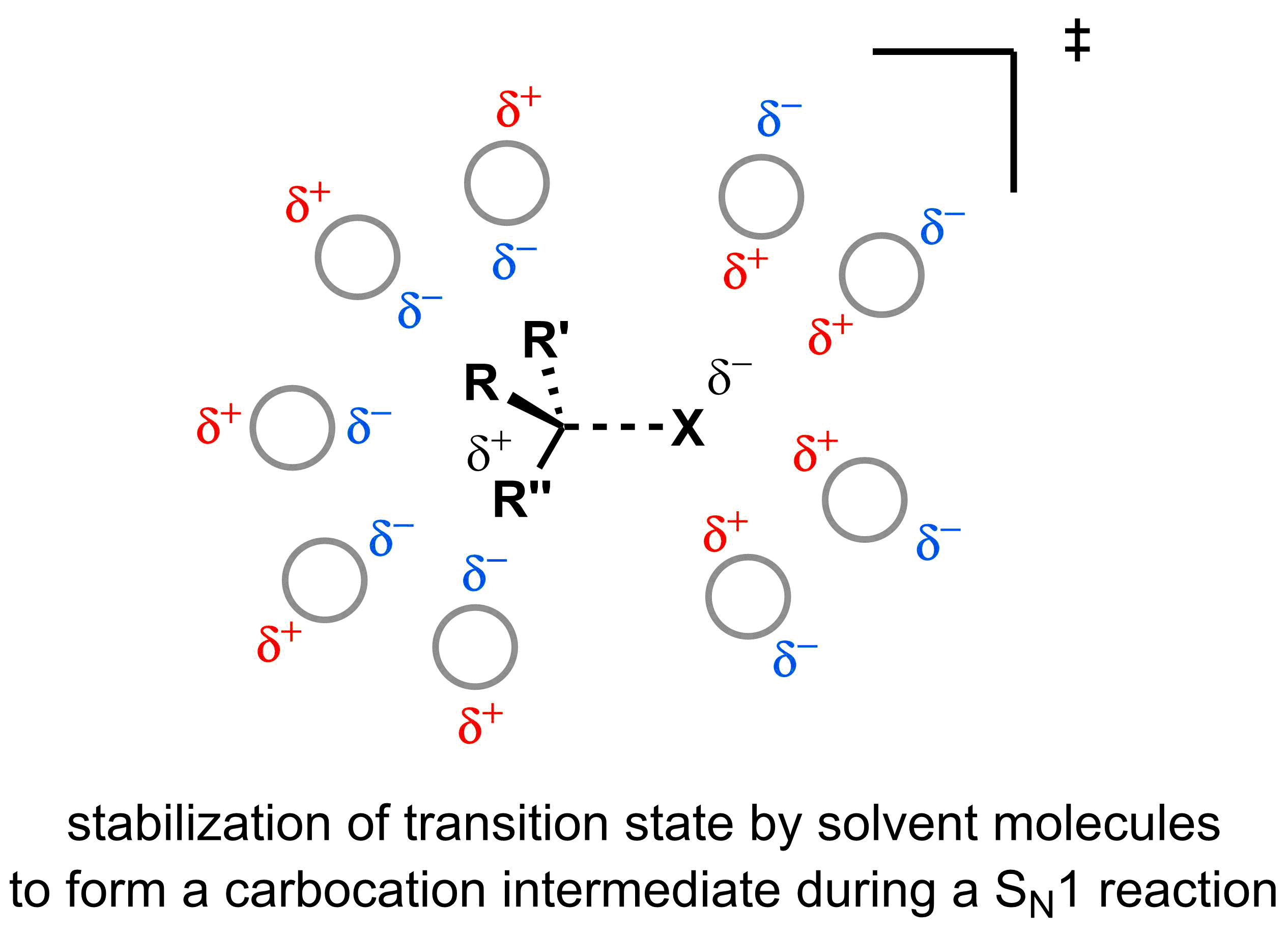
As the nucleophile concentration does not affect the rate of SN1 reactions, solvation of the nucleophile does not slow the reaction rate. This helps the SN1 mechanism dominate over the SN2 mechanism in polar protic solvents. Another factor that favours the SN1 mechanism in polar protic solvents is that hydrogen bonding can help accelerate the first step of an SN1 reaction. Polar protic solvents stabilize the transition state by forming hydrogen-bonds with the leaving group, which helps the formation of the carbocation intermediate and accelerates the rate determining step. Thus, SN1 reactions are typically most efficient in polar protic solvents.
Let's come back to our reaction from the beginning:
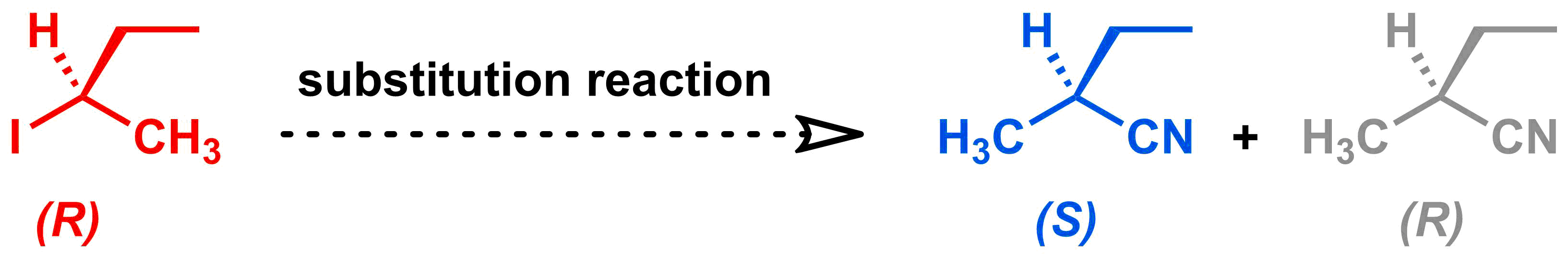
If we want the blue product only, then we want our reaction to proceed via the SN2 mechanism. The electrophile is 2°, so we need to take steps to help the SN2 mechanism dominate over SN1, since both mechanisms are possible with this electrophile. Complete the activity below to determine how we could set up the reaction to help the SN2 mechanism dominate:
After our reaction, we could analyze the product. If we get only the blue product, then we will know that our efforts to help the SN2 mechanism dominate were successful.
Guided:
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
Interactive:
Interactive (Intermediate):
Interactive (Challenge):