- The E/Z system of nomenclature can be used to name any alkene stereoisomer.
- An (E)-alkene has the two higher priority substituents on opposite sides of the double bond.
- A (Z)-alkene has the two higher priority substituents on the same side of the double bond.
There are three possible outcomes once priorities are assigned to each side of the alkene using the CIP rules introduced in the previous section; the two "high" ranking substituents are on opposite sides of the double bond, they are on the same side of the double bond, or at least one side of the alkene has identical substituents. If the high ranking substituents are on opposite sides of the double bond, it is called an (E)-alkene, from the German word entgegen meaning "opposite". If the high ranking substituents are on the same side of the double bond, it is called a (Z)-alkene, from the German word zusammen meaning "together". As opposed to cis/trans nomenclature, which can only be used for 1,2-disubstituted alkenes, the E/Z system of nomenclature can be applied to any alkene in options A and B.
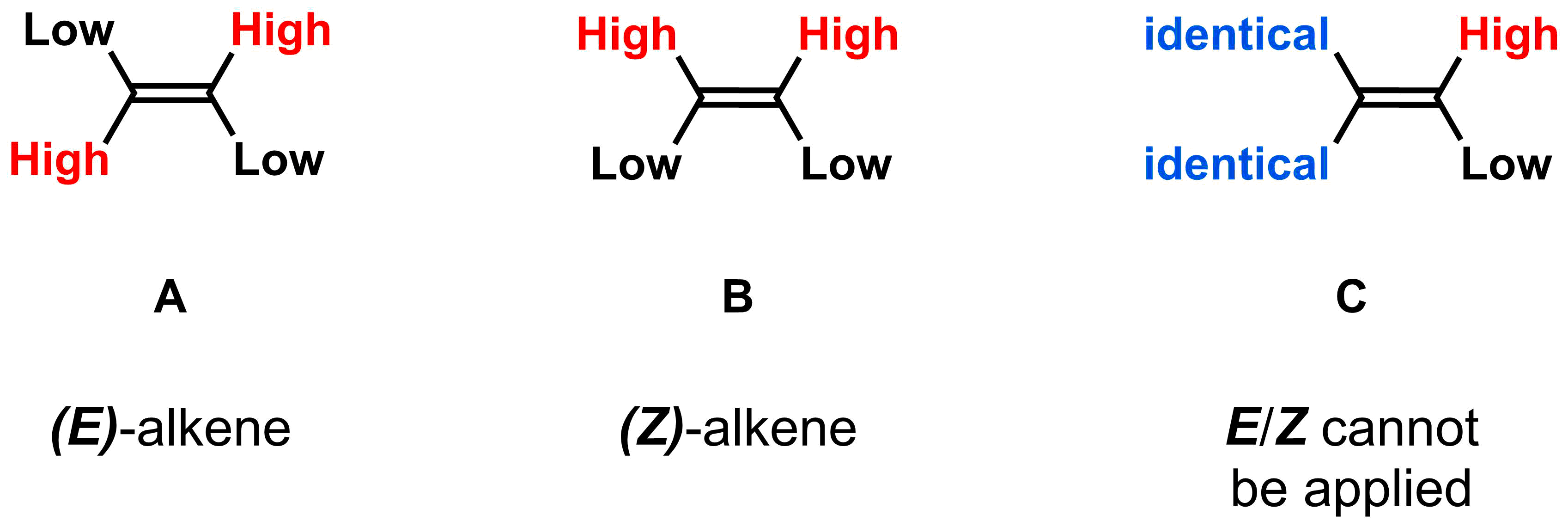
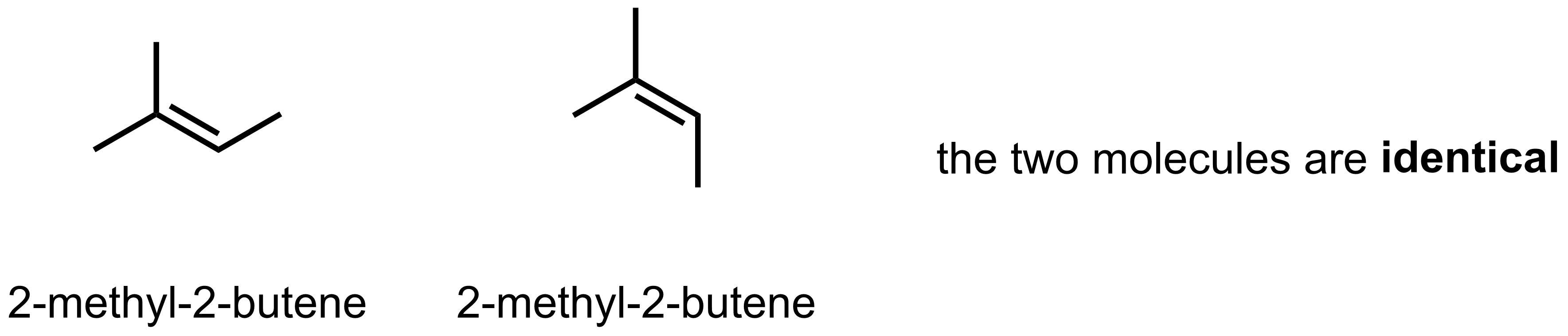
If at least one side of the alkene has identical substituents (option C), then E/Z nomenclature is not needed as switching the substituents on one side of the alkene does not result in a different alkene. For example, in 2-methyl-2-butene, there is no difference between the two molecules drawn below (they are not isomers). Therefore, additional nomenclature is not required and 2-methyl-2-butene is all that is required.
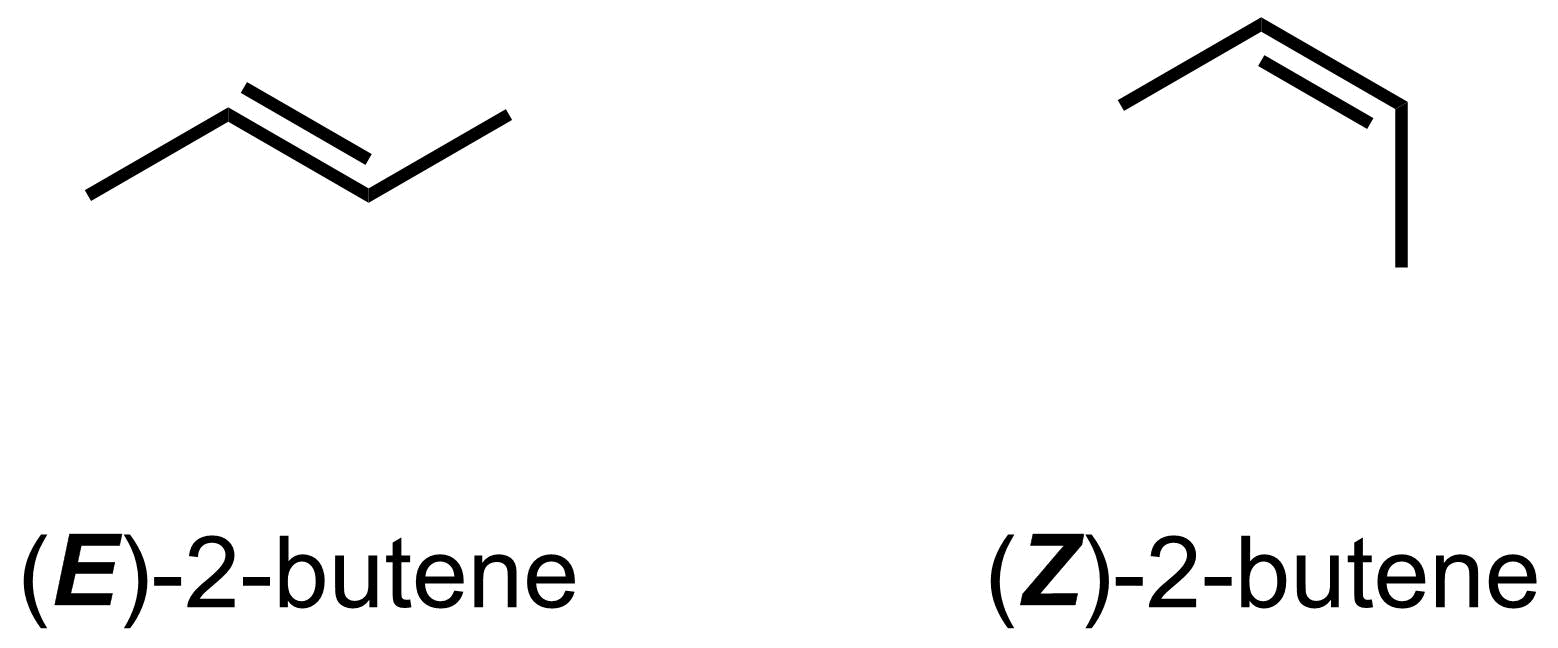
When naming a molecule with E/Z system, include an (E)- or (Z)- at the front of the name to indicate which isomer you have. For example, the left molecule below is (E)-2-butene, while the right molecule below is (Z)-2-butene.
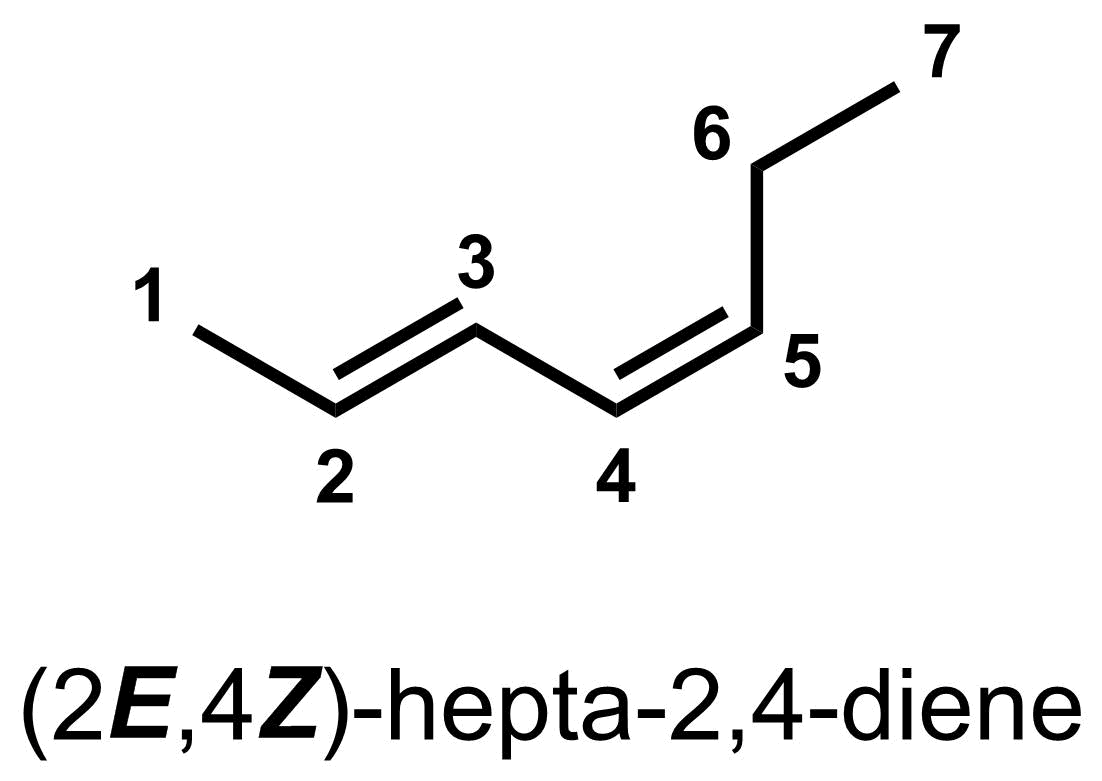
If you are naming a molecule with multiple double bonds that have E/Z configurations, then you should indicate whether each such double bond has the (E)- or (Z)-configuration. To do this, at the front of the molecule name, indicate the position (i.e. carbon number) and alkene configuration (i.e. (E)- or (Z)-) of each relevant double bond. For example, the double bond at carbon 2 below has the (E)-configuration while the double bond at carbon 4 has the (Z)-configuration, so we would name this molecule (2E,4Z)-hepta-2,4-diene.
In summary, to name a molecule with E/Z nomenclature:
1. Name the molecule using standard IUPAC rules
2. If there is one double bond with E/Z configuration, add (E)- or (Z)- to the front of the name to indicate which configuration you have
3. If there are multiple double bonds with E/Z configuration, indicate the position and orientation of each such double bond at the front of the name
For example:


