- Meso compounds have asymmetric centres, but are not chiral. This is because the mirror image of a meso compound is identical to the original molecule.
- All meso compounds have an internal plane of symmetry.
Every molecule that has only a single asymmetric centre will be chiral. This is not necessarily true for molecules that have multiple asymmetric centres. Take (1R,2S)-dimethylcyclohexane as an example. While this molecule has two asymmetric centres, it is superimposable with its mirror image and therefore, by definition, it is not chiral.
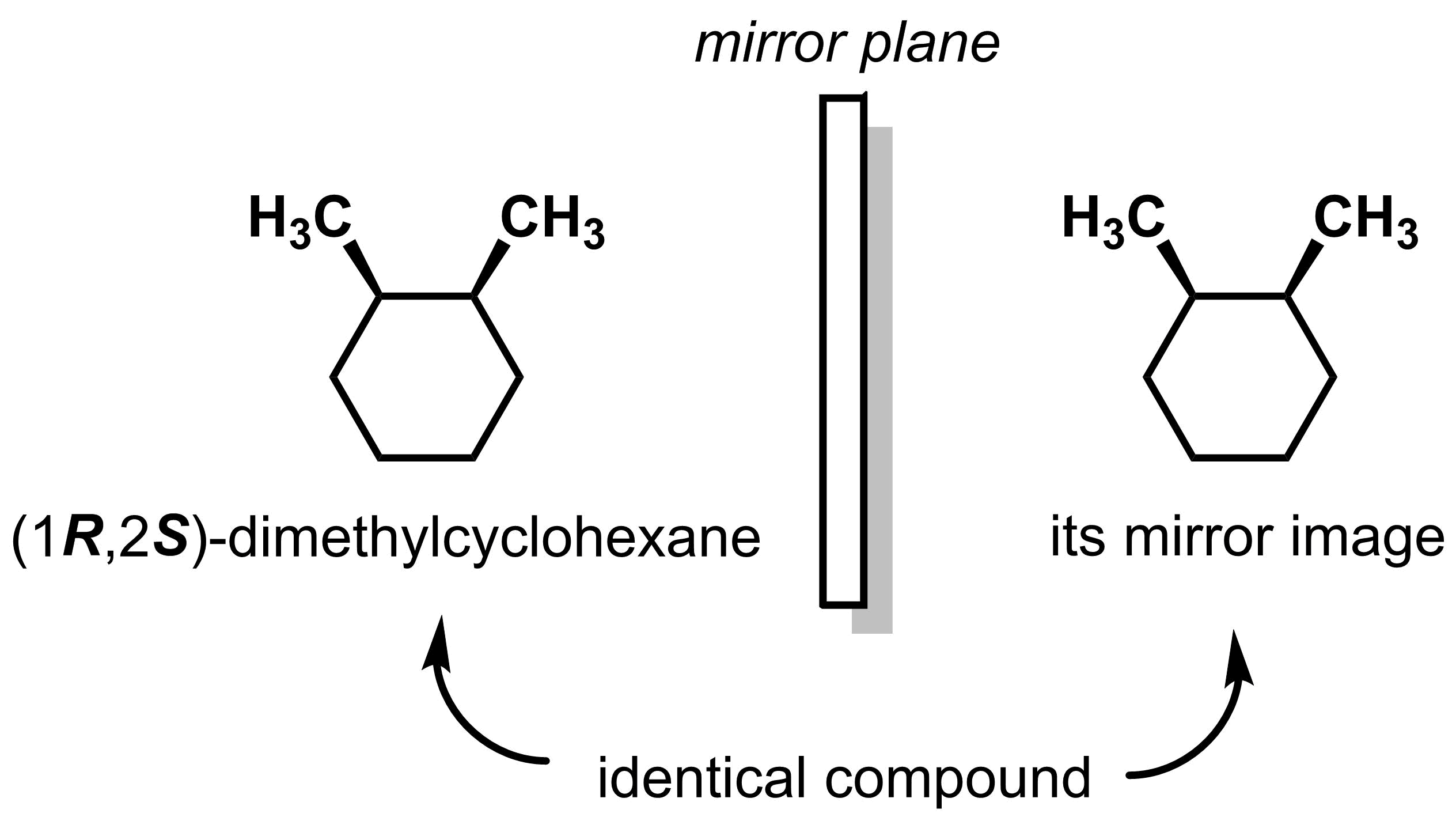
Molecules such as (1R,2S)-dimethylcyclohexane are called meso compounds, which are molecules that have asymmetric centres but are not chiral. Please note that the term meso is a property of a molecule and should not be used as a term of comparison. In the example above, (1R,2S)-dimethylcyclohexane is a meso compound. The relationship between (1R,2S)-dimethylcyclohexane and its mirror image is they are identical.
Meso compounds are one reason why the equation below has to be clarified with “at most”:
- number of stereoisomers is at most = 2(number of stereocentres)
For example, take 2,3-dibromobutane, which has 2 asymmetric centres. According to the equation above, this has, at most, the 4 stereoisomers drawn below.
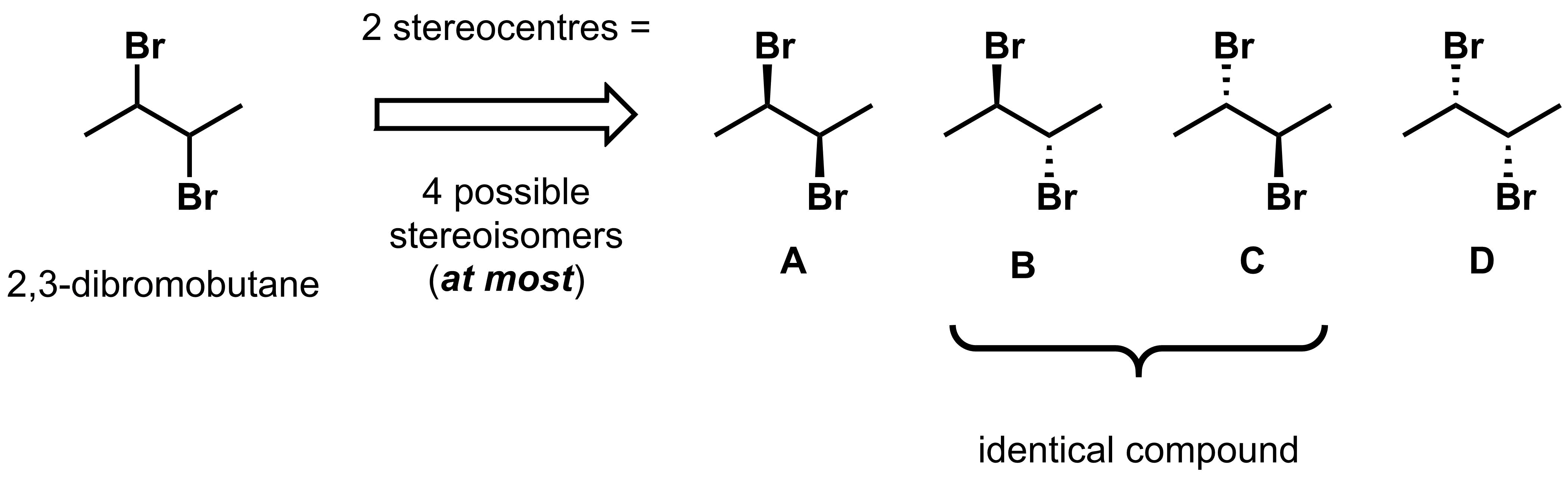
Analysis of the four reveals that there are only three distinct stereoisomers: A, B and D. C is not a distinct stereoisomer because it is identical to B, as shown below.
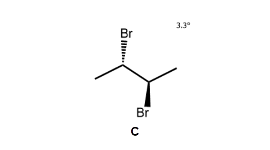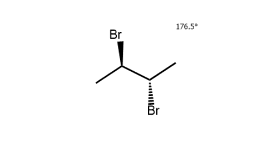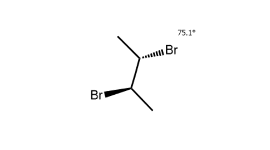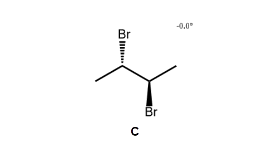
Since B has asymmetric centres but is achiral overall (it is identical to its mirror image, C), it is classified as a meso compound.
Interactive: