- Using a polarimeter, enantiomers can be distinguished by observing which direction the polarized light rotates.
- A sample with only one enantiomer is optically pure.
- A sample that rotates plane polarized light but is not optically pure is optically active.
- A sample with equal amounts of each enantiomer (racemic) is optically inactive.
The second way a chiral species can be differentiated is its interaction with plane polarized light. Plane polarized light is generated by passing light through a polarizer (a filter) and allows the electromagnetic wave to propagate in a single plane. Passing polarized light through a solution containing a chiral compound results in rotation of the plane. The angle of rotation can be measured by the detector. The instrument which allows to do this experiment is called a polarimeter.
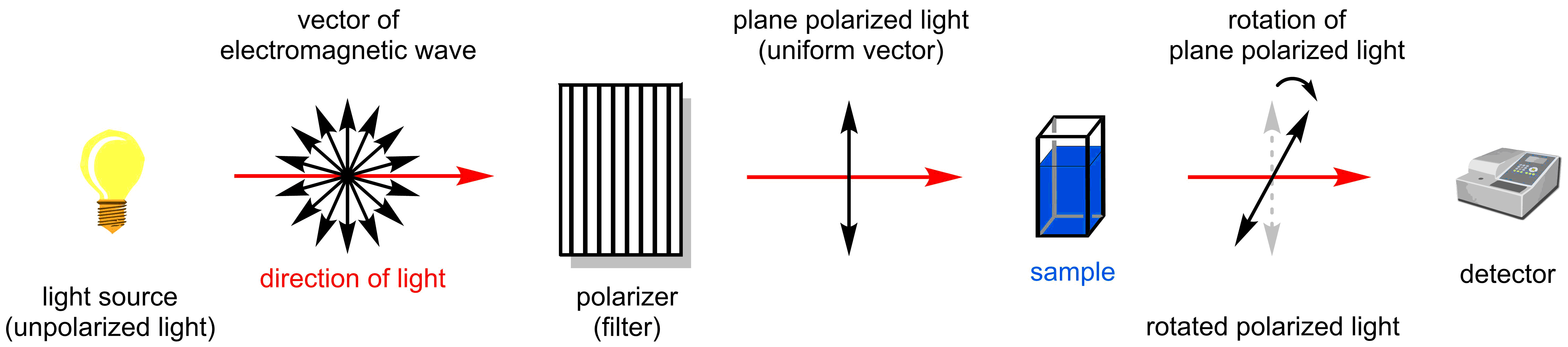
By convention, if the polarized light is rotated clockwise when viewing towards the light, the compound is assigned as the (+)-enantiomer or the (d)-enantiomer (dextrarotatory). If the anti-clockwise rotation is observed, the compound is assigned as the (−)-enantiomer or the (l)-enantiomer (levorotatory). Please note that there is no correlation between (R) or (S) and the direction light is rotated. To normalize the rotation of every sample, often specific rotations are reported, which is defined through the following equation:
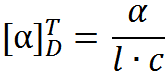
where [α] is the specific rotation for the sample taken at temperature T using the sodium D-line (wavelength of 489 nm), α is the measured optical rotation, l is the path length of the cell, and c is the concentration of the sample.
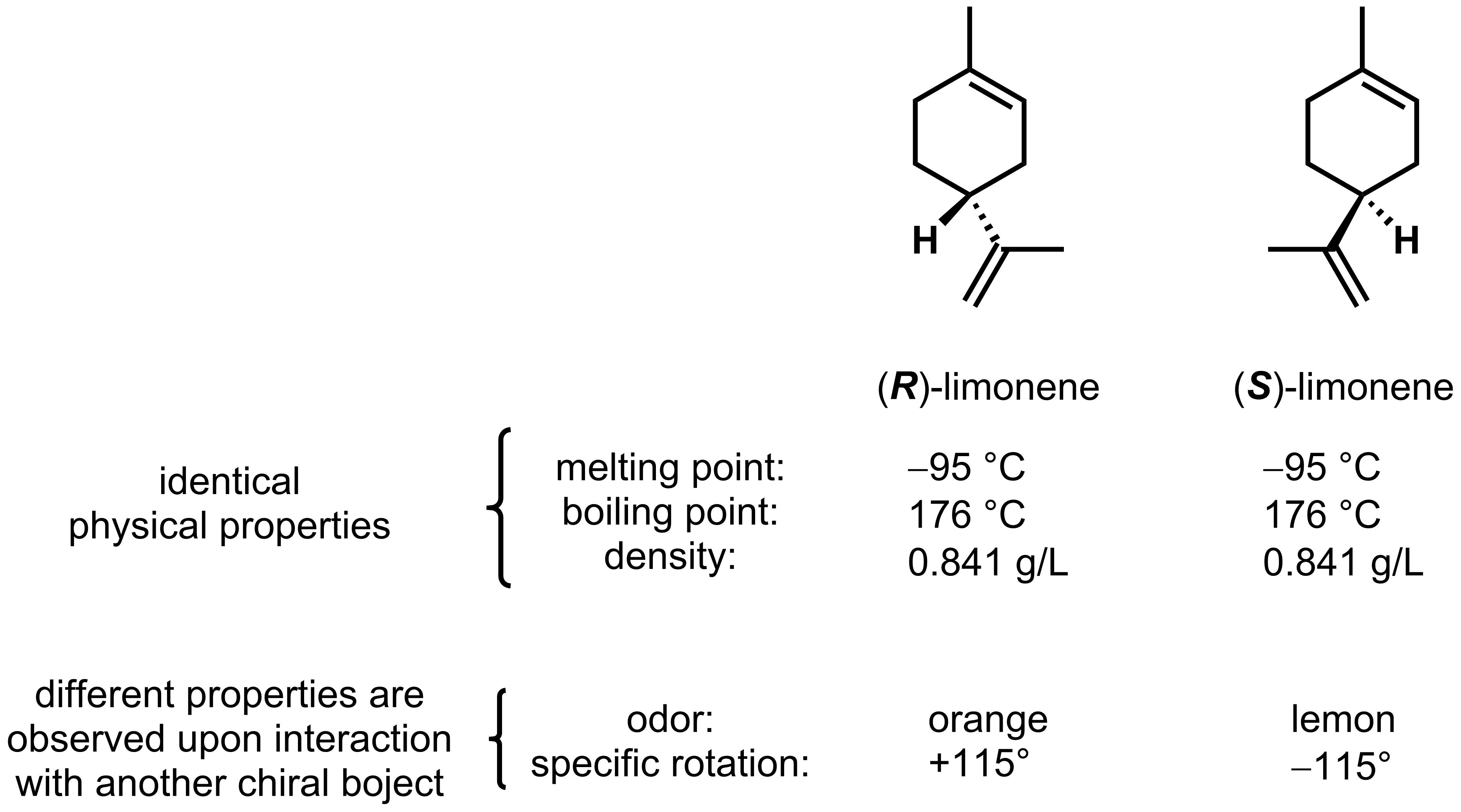
Enantiomers will rotate light in equal, but opposite directions, as seen with the specific rotation of the limonene enantiomers. If equal amounts of each enantiomer are present, the mixture is called racemic. Racemic mixtures will not rotate plane polarized light as each enantiomer rotates light an equal amount in opposite direction. Achiral molecules will also not rotate plane polarized light.
We can use the optical rotation of a mixture of two enantiomers, A and B, to help us determine how much of each enantiomer is present in the solution using the formula:
observed rotation = (%A/100)x(specific rotation of pure A) + (%B/100)x(specific rotation of pure B)
For example, using the data above, we can conclude that a solution consisting of 25% (R)-limonene and 75% (S)-limonene would have an observed rotation of:
observed rotation = (25/100)x(+115°) + (75/100)x(−115°) = −57.5°.
We can also describe solutions made up of a mixture of enantiomers using the enantiomeric excess (ee), which tells us how much of one enantiomer there is in excess of the other. The enantiomeric excess is defined as the percentage of the more abundant enantiomer minus the percentage of the less abundant enantiomer. An ee of 0 would indicate a racemic mixture (50% of each enantiomer). As an example, the solution above would have an enantiomeric excess of
The following terms are commonly used when referring to the data collected from a polarimeter:
- Optically pure: the sample matches the full expected optical rotation (only 1 enantiomer is present). For example, a reading of +115° will be obtained for an optically pure sample of (R)-limonene and −115° for (S)-limonene. An unequal mixture of (R)-limonene and (S)-limonene will result in an optically active sample (see below) with a optical rotation between +115° and −115°.
- Optically active: the sample rotates the plane polarized light, but it is contaminated with either smaller amounts of the other enantiomer or other diastereomers.
- Optically inactive: the sample does not rotate light. This means that either the sample is a racemic mixture (i.e. equal amounts of each enantiomer) or the molecules in the sample are achiral.
Interactive: