- (R) and (S) are labels that describe the configuration (i.e. 3D arrangement in space) of an asymmetric centre.
- To assign a configuration as (R) or (S), give each substituent a priority using the CIP rules, then draw a Newman projection with the #4 priority in the back.
- If the #1 → #2 → #3 priorities in the Newman projection proceed clockwise, it is assigned (R) configuration.
- If the #1 → #2 → #3 priorities in the Newman projection proceed counterclockwise, it is assigned (S) configuration.
Stereoisomers create an additional complication for nomenclature. For example, 2-bromobutane has one stereocentre, circled in red. The mirror image of 2-bromobutane is non-superimposable with the original molecule, and the two are different molecules. A new nomenclature is required to differentiate between the two structures.
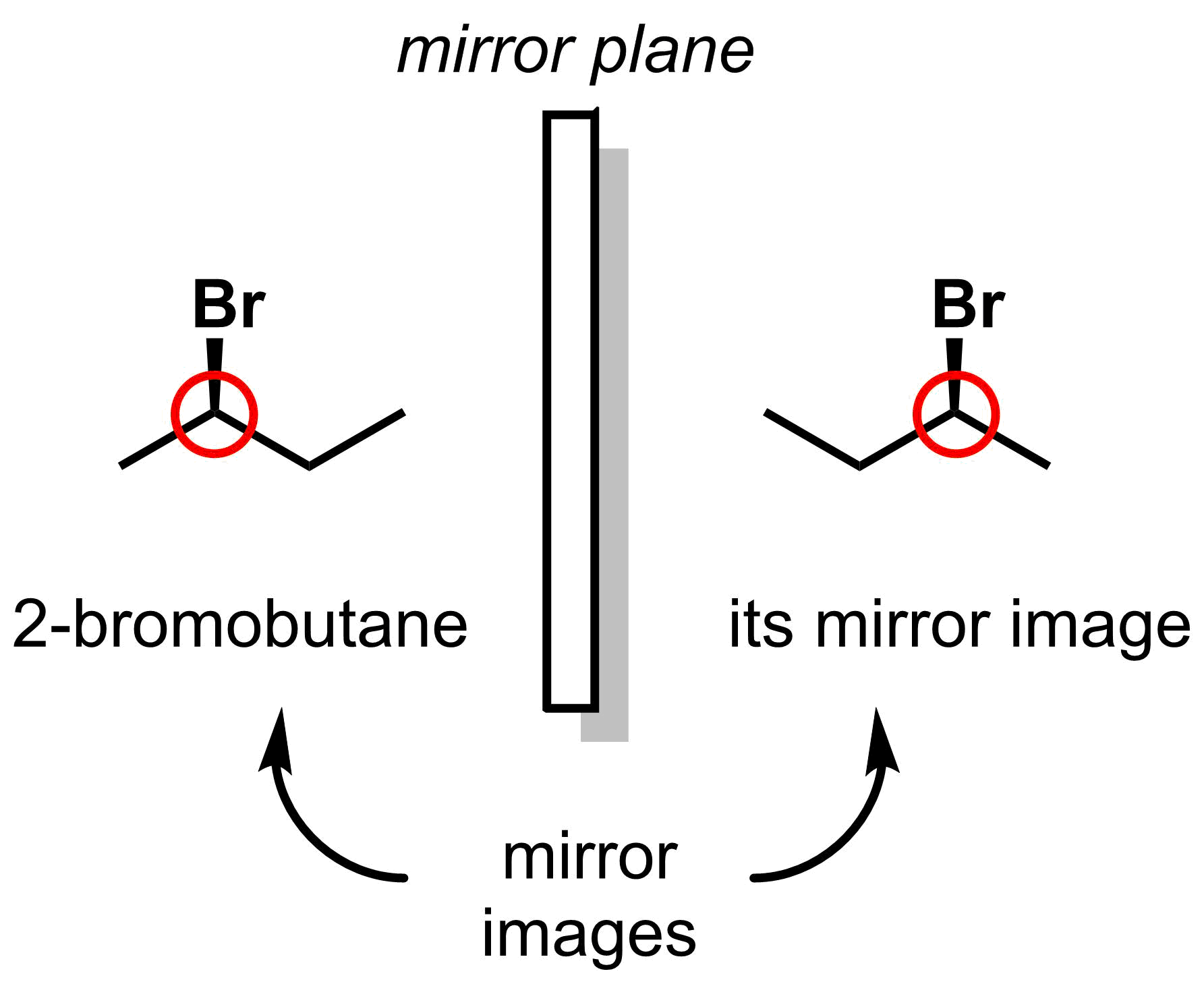
The new stereochemistry nomenclature that will be introduced in this section is R/S nomenclature, in which the configuration at the stereocentre will be assigned either as R or S. The following steps should be used to assign the correct configuration.
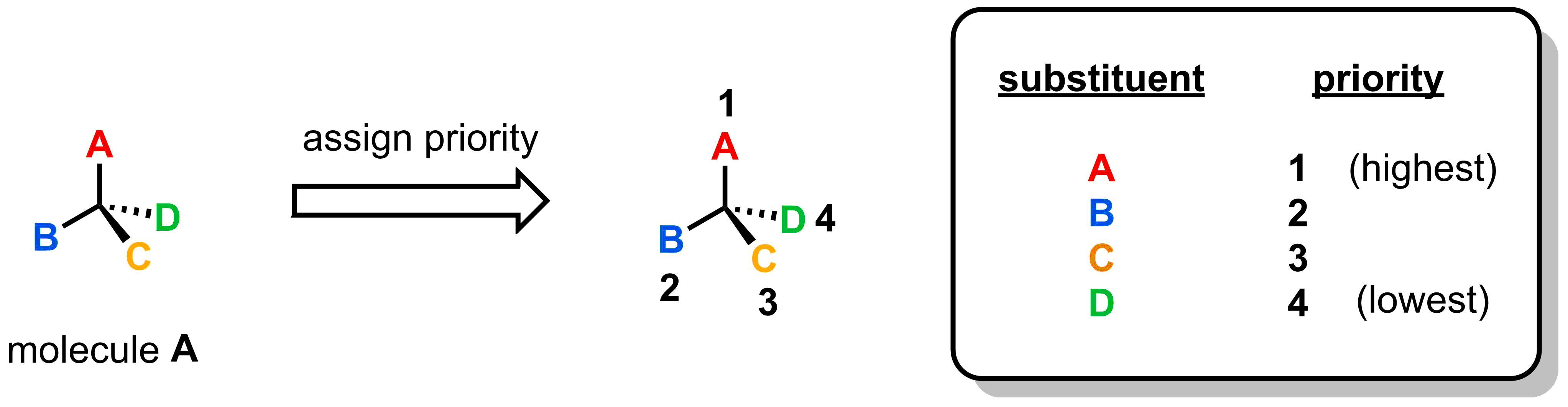
- Step 1: Assign priorities to the four different substituents attached to the stereocentre.
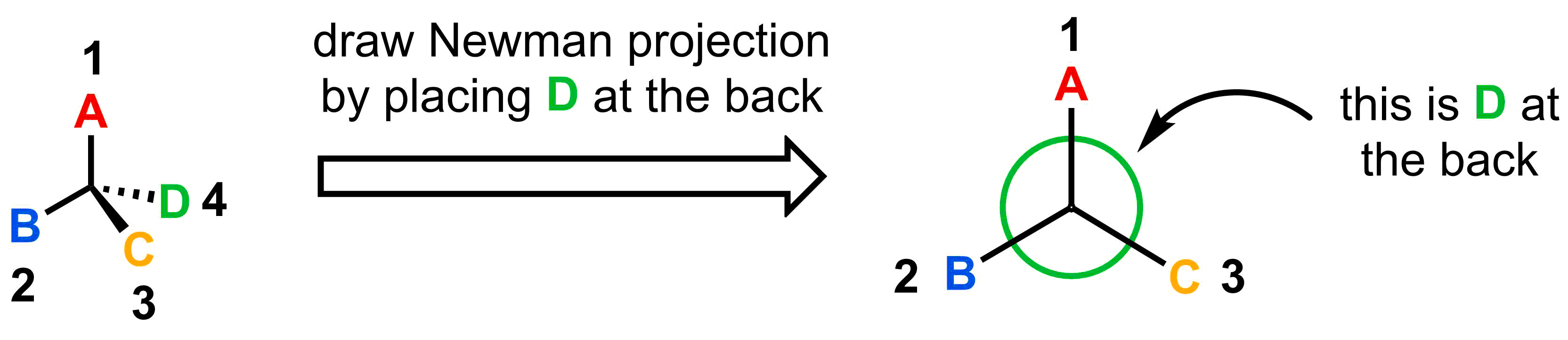
- Step 2: View the molecule from the stereocentre to the 4th priority substituent.
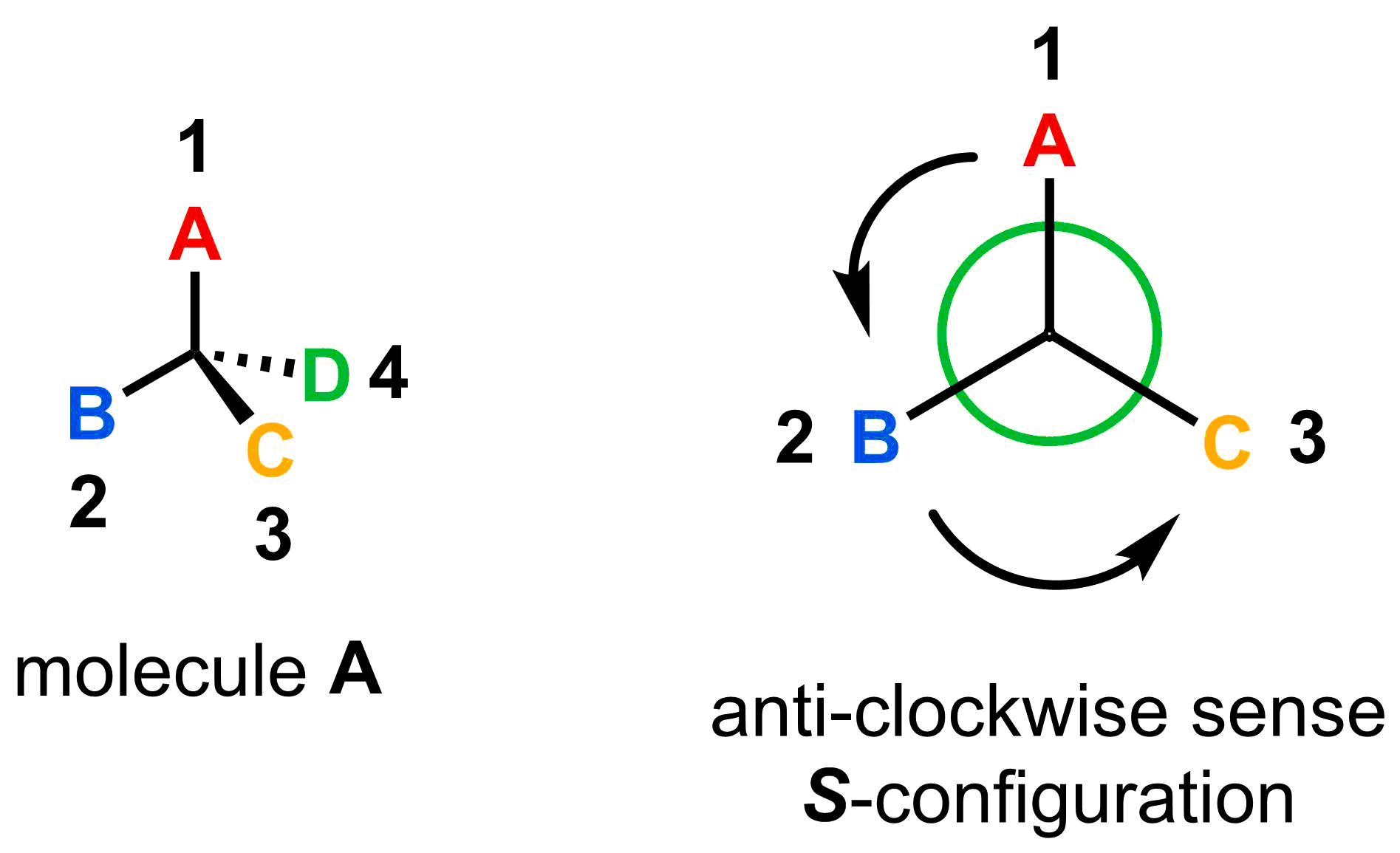
- Step 3: Determine whether the stereocentre is R or S.
Using the same CIP rules that we utilized in E/Z nomenclature (Part 4, section 1.3), assign priorities for each of the four substituents attached to the stereocentre. Assign from 1 (highest) to 4 (lowest).
Once priorities are assigned, view the molecule down the bond between the stereocentre and the 4th priority (make sure the stereocentre is in front). Redrawing the molecule as a Newman projection sighting along this bond is sometimes useful.
Draw arrows from the highest priority substituent (1) to the second highest priority substituent (2) and from the second to the third highest priority substituent (3). If the arrows are pointing in a clockwise sense, the stereocentre has an R configuration, after the Latin for right (rectus). If the arrows are pointing in a counterclockwise sense, the stereocentre has an S configuration, after the Latin for left (sinister).
Lets go back to the two isomers of 2-bromobutane you saw at the beginning of this section and apply this new nomenclature. Starting from the molecule shown below, the first step is to assign priorities. According to the Cahn-Ingold-Prelog rules, priorities can be assigned as follows: 1 = bromine, 2 = ethyl, 3 = methyl, and 4 = hydrogen. The next step is to sight along the steocentre-hydrogen axis, expressed as the Newman projection pictured below. Finally, drawing arrows from 1 to 2 and 2 to 3 is a clockwise motion, making this an R-stereocentre. Completing the name, this molecule is (R)-2-bromobutane.
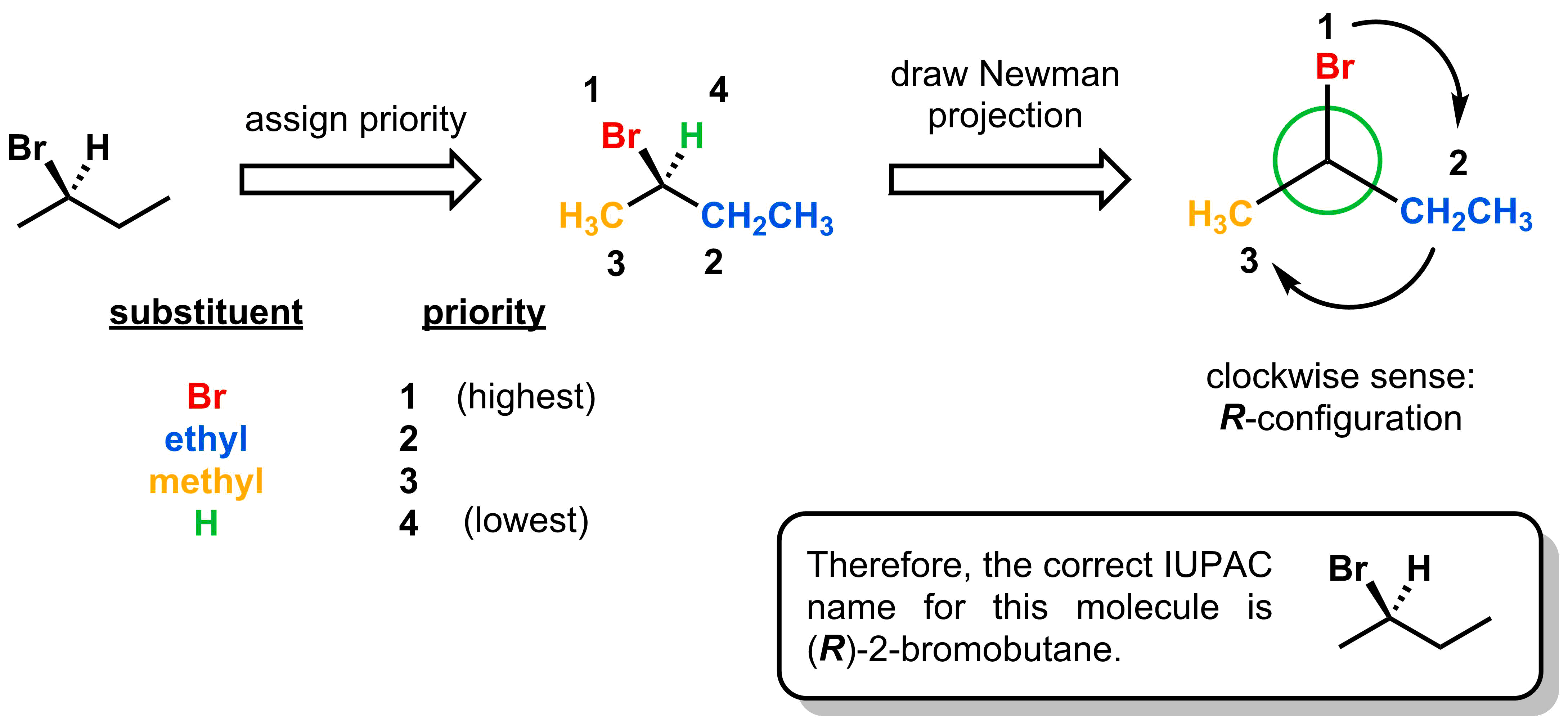
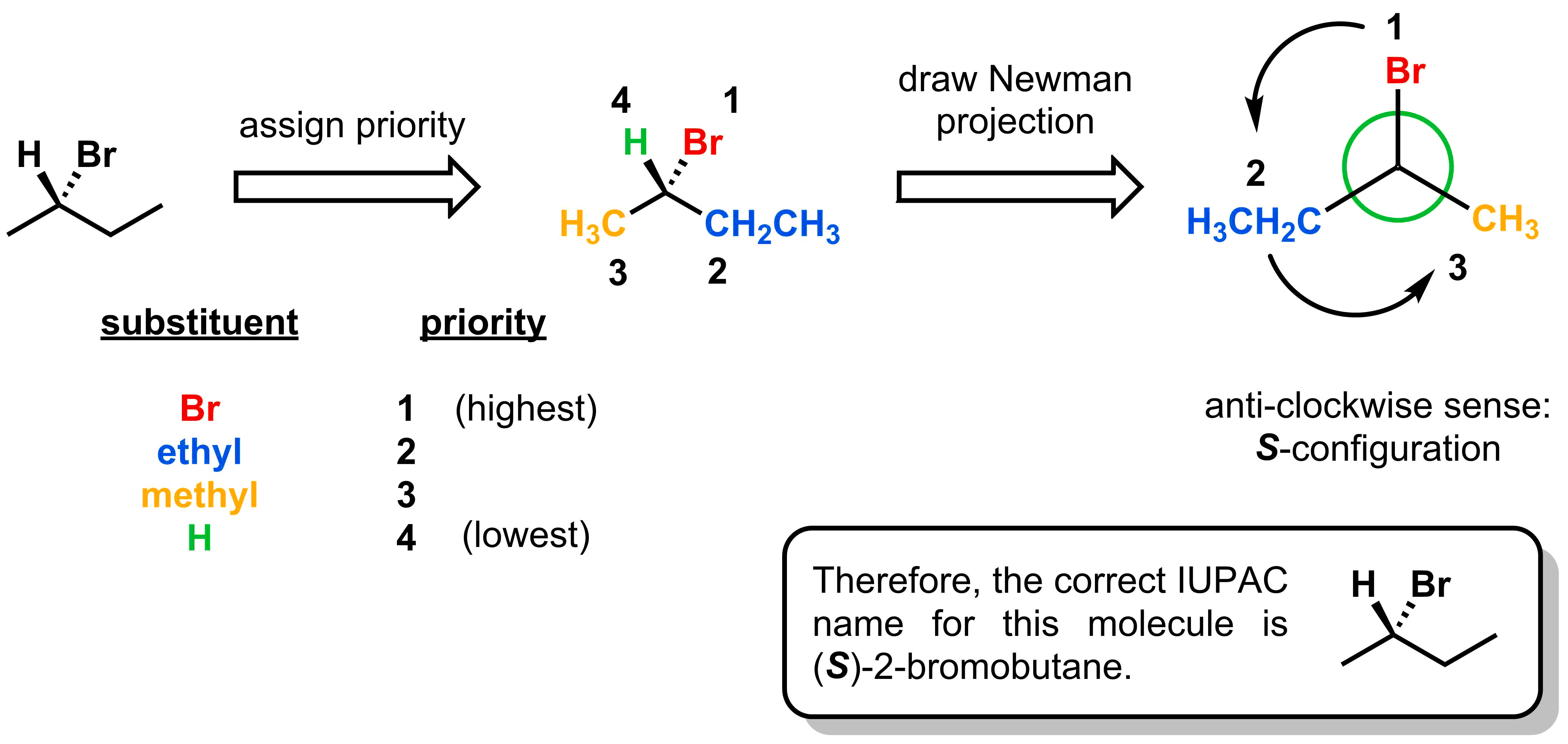
Following the same steps, the isomer shown below can be assigned an S-configuration.
Interactive: