- The rate of the generic chemical reaction
is defined as
- According to collision theory, in order for a chemical reaction to occur the reactants must collide with sufficient energy and the correct orientation.
- The rate of a chemical reaction increases when the concentration, pressure, and/or temperature are increased.
- A rate law shows the relationship between the rate of a reaction and the concentrations of the reactants
.
- The rate constant, k, is a proportionality constant that shows how the rate relates to the concentrations of the reactants in a rate law.
- Rate laws can be determined experimentally using the method of initial rates by varying the concentration of a reactant and measuring how it affects the initial rate of the reaction.
The rate of a chemical reaction is a measure of how quickly the concentrations of the reactants and products change over time. For the generic reaction
the rate is defined as
(Equation 1).
Review: Wondering what ![\frac{d[X]}{dt}](https://latex.codecogs.com/svg.latex?\inline&space;\frac{d[X]}{dt}) means? Expand this section for a review of derivatives.
means? Expand this section for a review of derivatives.
Calculus is a very powerful tool for describing chemical phenomena. In CHEM 123, we generally avoid using calculus since it is not a pre-requisite for the course. However, there are two instances in kinetics where calculus is essential for our discussion. Our first instance, here, requires a very basic understanding of a derivative.
Consider the two graphs of the concentration of chemical X, [X], versus time, t. The slope at any point on one of these graphs tells us the rate (how quickly [X] changes over time) of the chemical reaction at that point.
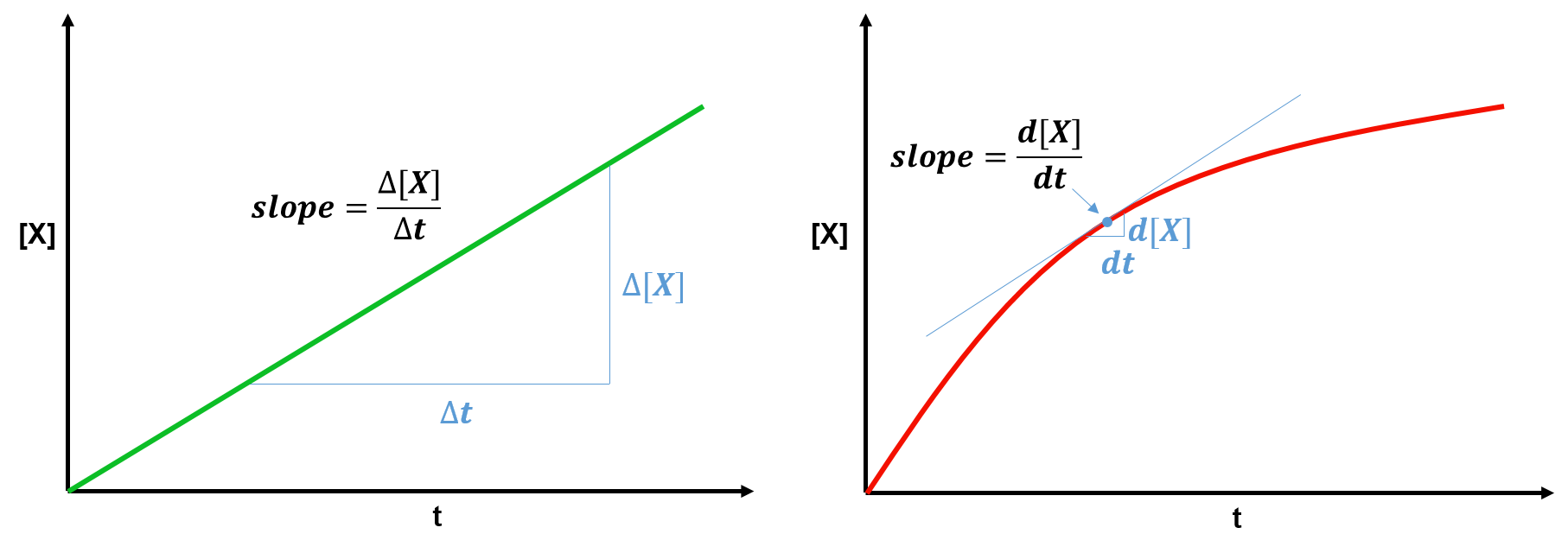
The plot on the left is a straight line, so measuring the rate (slope) is straightforward. The rate (slope) here is simply at all points. However, when we have a curve, like on the right, the rate (slope) is continually changing. Taking smaller
and
measurements close to the point of interest (e.g. the blue point on the right graph) can give an estimate of the slope (rate) at that point. '
' represents a relatively large change. When these
and
measurements are infinitely small, they are called
and
.
is called the derivative and gives the instanteous slope at a particular time,
. For example, the derivative at the blue point gives the instantaneous slope (the slope of the tangent line shown in blue) at this point.
The derivative terms in the main text above (,
,
, and
) reflect the instanteous rate that each of these concentrations are changing at a particular time, t.
The rate is always quoted as a positive value. Notice that there are negative signs in front of the two terms relating to the reactants ( and
). Since reactants are disappearing, the concentrations of [A] and [B] are decreasing and thus
and
are both negative. These derivatives are thus multiplied by -1 to give a positive number. The product terms,
and
, are already positive.
Also, note that the rate for each individual species is multiplied by , where
is the coefficient for that term in the balanced reaction. To understand why this is necessary, consider an example with the reaction
.
The overall rate must be the same no matter which reactant or product we track. Based on the stoichiometry of the reaction, two molecules are produced every time one
molecule is produced. Thus,
appears at half the rate that
appears:
. The overall rate of this reaction is thus
.
Try it: Select the point on the graph below that corresponds to the highest rate:
According to Collision Theory, in order for a chemical reaction to occur, the reactants must (1) collide with (2) sufficient energy and (3) the correct orientation. Thus, we'd expect anything that increases the number of collisions or the energy of those collisions would increase the rate of a chemical reaction. Complete the activity below to explore how pressure, concentration, and temperature affect the rate of a chemical reaction and why:
Since the rate of the reaction depends on the concentration of the reactants, the same rate defined in Equation 1 for reaction
can also be written as
where the values of and
depend on the particular reaction or
(Equation 2)
where is a proportionality constant called the rate constant. Equations that relate the rate of a reaction directly to the concentrations of the reactants, like Equation 2, are called rate laws.
We can determine the values of ,
, and
experimentally by measuring the rate with various starting concentrations of
and
. As soon as
and
are added, their concentrations will begin to decrease as they react and the rate of the reaction may also change (as described above). Thus, it's important to measure the reaction rate immediately after
and
are added when the exact concentrations are known. This is called the Method of Initial Rates and can be used to determine the rate law for many reactions.
When asked to determine a rate law using the Method of Initial Rates in CHEM 123, experimental data that includes initial rates and reactant concentrations will be provided for you. For example, consider the reaction
with experimental data (at 298 K)
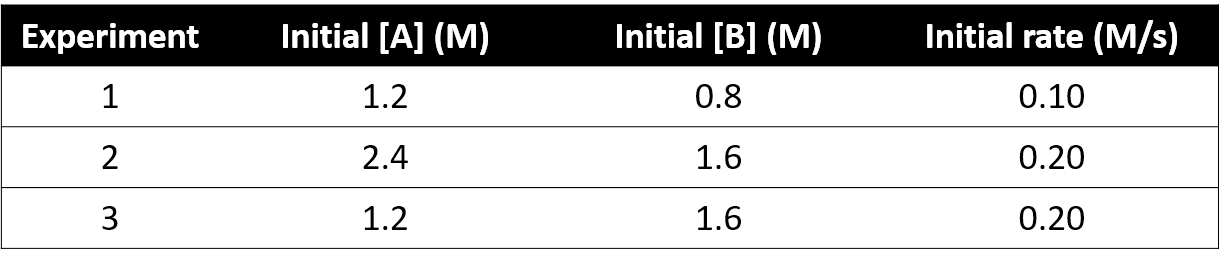
By comparing experiments 1 and 3, we can see that doubling [B] while holding [A] constant doubles the initial rate. We can deduce that the rate must be proportional to [B]1. Comparing experiments 2 and 3 shows us that when we halve [A] but hold [B] constant, the rate does not change. This shows that the rate does not depend on [A]. Thus, the rate law for this reaction is .
In this particular example, all the numbers differed by exactly a factor of 2 so we could work out the rate law simply. The numbers, though, may not always be so simple. We'll solve this same question again in a more systematic way that you could apply if it wasn't straightforward to see the relationship between the concentrations and the rate simply by looking at the table.
At the same temperature, the rate constant, is the same. Solving Equation 2 for
gives
(Equation 3).
All three experiments above were conducted at the same temperature, so all have the same value. Thus,
where the subscripts 1, 2, and 3 indicate the experiment number. The easiest way to solve for and/or
is to choose two experiments where one of these terms cancels out. Notice that in both experiments 1 and 3
while the concentration of
changes. Thus, comparing experiments 1 and 3 will allow us to isolate how changing
affects the rate.
.
The terms are the same on both sides, so cancel out to give
.
Rearranging this equation gives
and thus since
it must be that
.
Experiments 2 and 3, where changes but
is held constant, give
via a parallel method. First,
and
so the two terms cancel out. Rearranging gives
and so is
.
The rate law for this example is thus
.
Note that with this information we could also solve for by plugging in the data from any of the 3 experiments to Equation 3. The units of
vary depending on how many concentration terms are in a rate law to ensure that the overall rate is always in units of concentration per time.
In a rate law, the exponent for each concentration term dictates the order with respect to that reactant. The sum of all exponent terms in a rate law dictates the order of the overall reaction. The order is helpful in understanding how quickly the rate of a reaction changes as each concentration term changes. The rate law determined above to demonstrate the Method of Initial rates is 0th order with respect to A, 1st order with respect to B, and 1st order overall.
View solution:
is 2nd order with respect to A, 3rd order with respect to B, 1st order with respect to C, and 6th order overall.
Interactive: