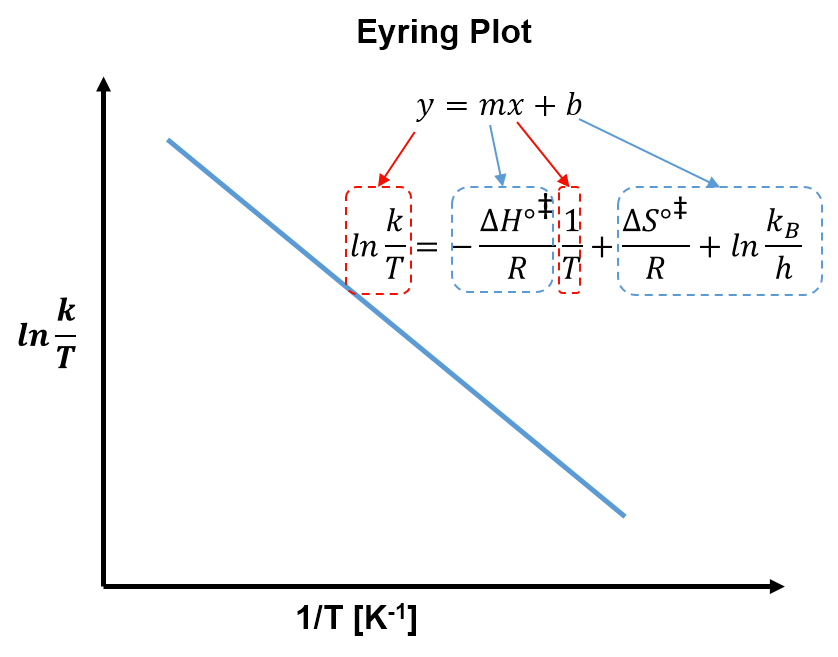
- The linear form of the Eyring equation is
- A plot of
versus
gives a straight line with slope
and y-intercept
.
While useful and still widely used, the Arrhenius equation,
(Equation 1),
is somewhat unsatisfying in that it relies on an experimentally determined proportionality constant, A, for each reaction. The Eyring equation (Equation 2),
(Equation 2),
is an analogous equation for the free energy of activation, ‡, instead of the activation energy, and eliminates the need for this reaction-specific proportionality constant. In Equation 2, R is the universal gas constant (
), T is the temperature (in Kelvin), h is the Planck constant (
), and kB is the Boltzmann constant (
). The derivation of the Eyring equation will not be examined in CHEM 123, but is included below for your interest.
Derivation: Expand this section to see the derivation of Equation 2.
Video derivation:
Text-based derviation:
We can derive most of Equation 2 with concepts from CHEM 123, though, as you'll see below, the details of one step in the derivation will only come in later chemistry courses.
Imagine the reaction
with the reaction coordinate diagram:
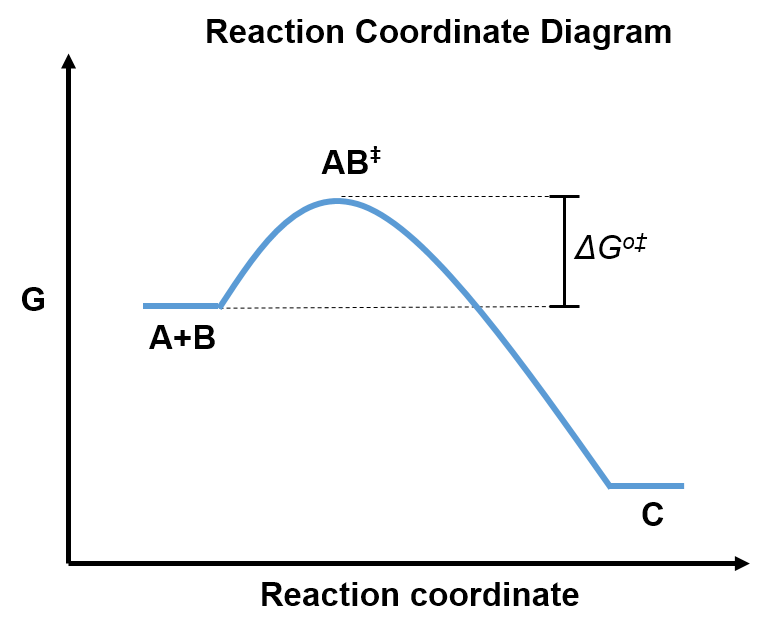
We can consider this reaction in two stages: first, the reactants (A and B) form the transition state (), then the transition state transforms into the product (C), such that
where is the rate constant that describes the how quickly the transition state transforms into the product, and
is the equilibrium constant between the the reactants and transition state given by
(Equation 3).
Considering the second stage of this process,
,
the products are formed at the rate that the transition state transforms into product such that
.
Solving Equation 3 for gives
and thus the rate is
(Equation 4).
Since is an elementary reaction, we could also describe the rate as
(Equation 5).
By comparison of Equations 4 and 5, it must be that
(Equation 6).
Now consider the first stage of this reaction:
.
Since the standard Gibbs free energy difference relates to the equilibrium constant (see Part 1 Section 4.2), here the free energy of activation, , relates to the equilibrium constant,
, between the reactants and transition state by
such that
.
Equation 6 thus can also be written as
(Equation 7).
Finally, the rate constant, , for the the transformation of the transition state to products is given by
.
The details of this step are beyond the scope of CHEM 123. Substituting this new term for into Equation 7 gives the Eyring equation
(Equation 2).
The standard free energy of activation relates to the standard enthalpy of activation, , and the standard entropy of activation,
, by
.
Thus, the Eyring equation (Equation 2) can also be written as
(Equation 8).
Dividing both sides of Equation 8 by T gives
and taking the natural logarithm of both sides and rearranging gives
(Equation 9).
Equation 9 is a useful form of the Eyring equation because a plot of versus
gives a linear plot with slope
and y-intercept
.
That is, Equation 9 can be used to calculate and
(and thus also
) when given temperature and rate constant data.
Interactive: