- The largest barrier in a kinetic process is called the rate-determining step, as it is the slowest step of a chemical reaction and will determine the speed (rate) at which the overall reaction proceeds.
- If an overall reaction occurs over two elementary steps, where the first step is rate-determining, then the overall rate of the reaction is equal to the rate of the first elementary step.
To help with our discussion of multistep reactions, it is first important to recall several key terms. Usually, chemical processes are described using overall reaction equations, which describe the starting materials and products, including their stoichiometry. However, the overall reaction equations do not detail the individual steps that it takes for the reactants to reach the products. Each individual step is called an elementary reaction, and is defined as a process that occurs in a single step and passes through a single transition state. For example, let’s examine the overall reaction process below
Overall reaction
This reaction equation details how much starting material is needed to go to products, but it gives no information about how the reaction occurs. The reaction may go through a single-step process, in which the overall and elementary reactions are the same
Overall reaction
Elementary reaction
Alternatively, it may require a multi-step process. For example, in the example below there are two elementary steps.
Overall reaction
Elementary reactions
The sum of these elementary reactions is equal to the overall reaction. In the example above, there is a new entity, C, that is both formed and consumed during the course of this reaction. In this example, C is a reaction intermediate, which is a molecular entity with an appreciable lifetime that is formed from the reactants and reacts further to give the products of a chemical reaction. The diagrams below show two different possible reaction coordinate diagrams for this two-step reaction. Note that (1) there is a single transition between each of the two elementary steps and (2) the intermediates are located in energy minima (meaning that they have an appreciable lifetime).
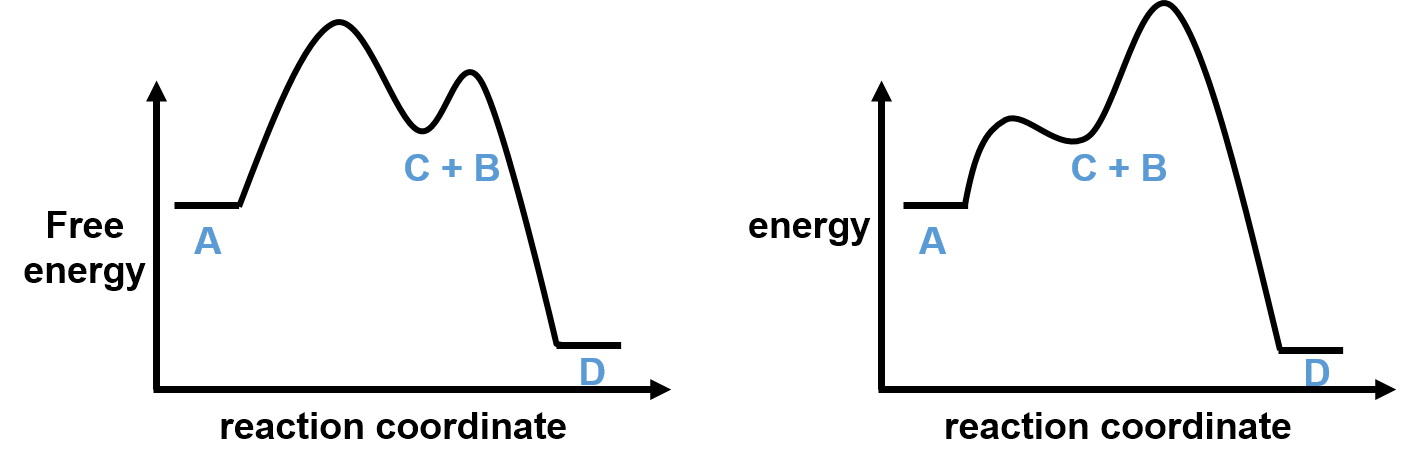
The relative heights of the energy barriers for each of the elementary steps for these two reaction coordinate possibilities are very different; in the example on the left, there is a larger barrier for the first elementary step while on the right the second barrier is larger. The largest barrier in a kinetic process is called the rate-determining step, as it is the slowest step of a chemical reaction and will determine the speed (rate) at which the overall reaction proceeds. Thus, in the example on the left, the first step is the rate determining while the second step is rate determining on the example on the right. As you will see below, the kinetics for these two possibilities will be very different.
Let’s first analyze the kinetics for a multi-step reaction in which the first step is rate determining. As we are now referring to rates, we will add rate constants to each of the elementary reactions.
If the first step is rate determining, the rate of the first step must be slower than the second. Thus,
By definition, everything after the rate determining step is faster and will not factor into the overall reaction rate. The rate of the overall reaction is simply the rate of the first elementary step, or
Let’s now examine two other possible reaction coordinate diagrams for this process below.
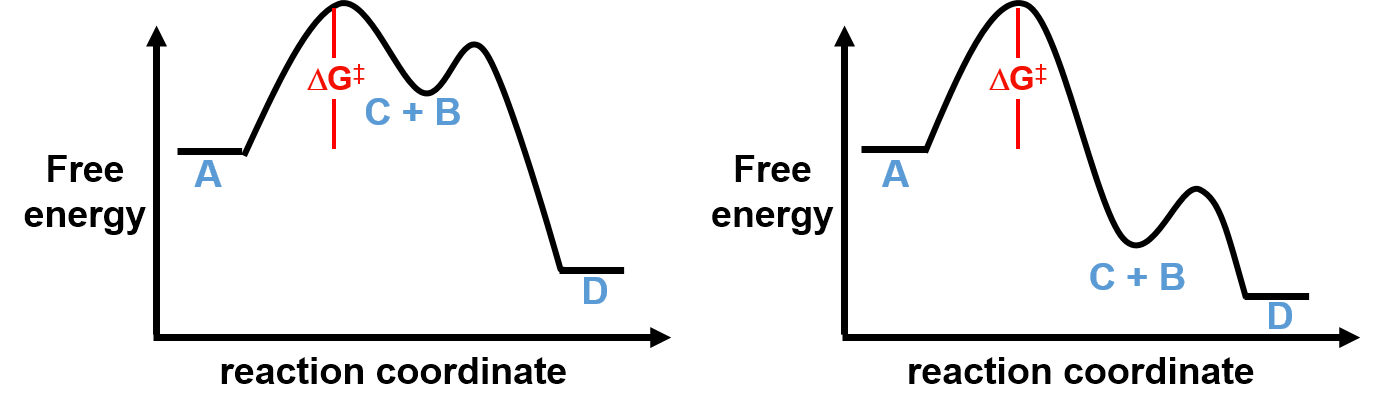
In both cases, the first step is the rate determining step and the height of the barrier is the same (i.e. the magnitude of ΔG‡ in the first steps are identical). Even though the relative energetics of the intermediate and activation energies for the second elementary steps are very different, the rates of both processes will be the same (Rate = k1[A]) because the differences occur after the rate determining step.
Interactive: