- If the second elementary reaction in a two-step mechanism is rate-determining, the rate of the overall reaction is equal to the rate of the second elementary step.
- At equilibrium, the rate of the forward and reverse elementary reactions are equal.
- A final rate law should not include intermediates.
The previous section described multi-step processes in which the first step is rate determining. Let’s now examine processes where the second step is rate determining, as it is for the the two-step reaction
with reaction coordinate diagram
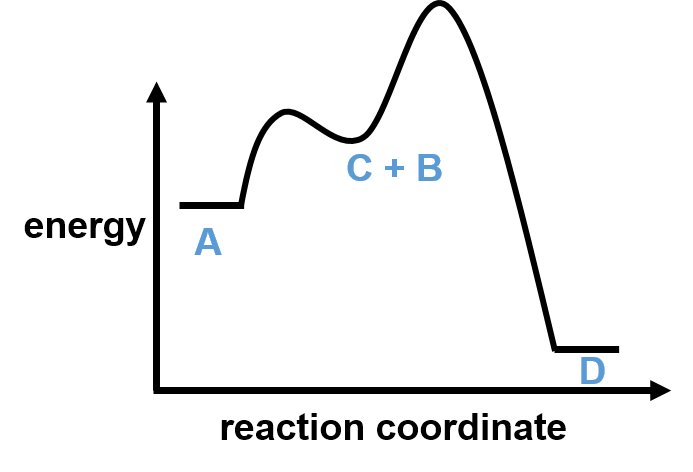
If the second step is rate determining, then there is enough energy in the system to go both from A to C and back from C to A. This means we can rewrite the rate equation for the first elementary step as an equilibrium while the second step remains unidirectional.
The rate of the reaction can be expressed in terms of the rate determining step:
This brings up a challenge as the concentration of intermediate C is difficult to directly measure. It would be much more powerful to express the rate in terms of only reactants. To do this, we need to find a new expression for [C]. One option to do this is to use the fact that the first elementary step is in equilibrium. When species are in equilibrium, the rate of the forward step (k1[A]) is the same as the reverse (k-1[C]), or
Solving for C affords
Substituting for [C] into the rate equation gives
In the next section, we will see another method to examine processes in which the second step is rate determining.
Interactive: